R409K mutation prevents acid-induced aggregation of human IgG4
- PMID: 32182240
- PMCID: PMC7077836
- DOI: 10.1371/journal.pone.0229027
R409K mutation prevents acid-induced aggregation of human IgG4
Abstract
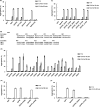
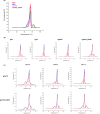
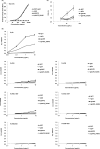
Human immunoglobulin G isotype 4 (IgG4) antibodies are suitable for use in either the antagonist or agonist format because their low effector functions prevent target cytotoxicity or unwanted cytokine secretion. However, while manufacturing therapeutic antibodies, they are exposed to low pH during purification, and IgG4 is more susceptible to low-pH-induced aggregation than IgG1. Therefore, we investigated the underlying mechanisms of IgG4 aggregation at low pH and engineered an IgG4 with enhanced stability. By swapping the constant regions of IgG1 and IgG4, we determined that the constant heavy chain (CH3) domain is critical for aggregate formation, but a core-hinge-stabilizing S228P mutation in IgG4 is insufficient for preventing aggregation. To identify the aggregation-prone amino acid, we substituted the CH3 domain of IgG4 with that of IgG1, changing IgG4 Arg409 to a Lys, thereby preventing the aggregation of the IgG4 variant as effectively as in IgG1. A stabilizing effect was also recorded with other variable-region variants. Analysis of thermal stability using differential scanning calorimetry revealed that the R409K substitution increased the Tm value of CH3, suggesting that the R409K mutation contributed to the structural strengthening of the CH3-CH3 interaction. The R409K mutation did not influence the binding to antigens/human Fcγ receptors; whereas, the concurrent S228P and R409K mutations in IgG4 suppressed Fab-arm exchange drastically and as effectively as in IgG1, in both in vitro and in vivo in mice models. Our findings suggest that the IgG4 R409K variant represents a potential therapeutic IgG for use in low-effector-activity format that exhibits increased stability.
Conflict of interest statement
Hiroshi Namisaki, Seiji Saito, Keiko Hiraishi, Tomoko Haba, Hideaki Yoshida, Shigeru Iida, and Nobuaki Takahashi are employed by Kyowa Kirin Co., Ltd. These do not alter the authors’ adherence to PLOS ONE policies on sharing data and materials. There is a patent application WO2006/033386 which is relevant to the research disclosed at this manuscript. There are no further patents, products in development or marketed products associated with this research to declare.
Figures

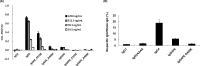
References
-
- Poiron C, Wu Y, Ginestoux C, Ehrenmann F, Patrice D, Lefranc MP. IMGT/mAb-DB: the IMGT database for therapeutic monoclonal antibodies. JOBIM 2010;13.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

