Impaired Anti-Tumor T cell Response in Hepatocellular Carcinoma
- PMID: 32182707
- PMCID: PMC7139707
- DOI: 10.3390/cancers12030627
Impaired Anti-Tumor T cell Response in Hepatocellular Carcinoma
Abstract
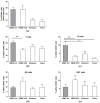
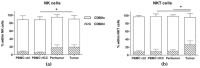
Different subsets of lymphocytes have the capacity to promote or counteract the progression of solid cancers, including hepatocellular carcinoma (HCC). Therefore, to determine the infiltrative ability and functional status of major immune cell subtypes into tumor may lead to novel insights from the perspective of immunotherapy. After obtaining single cell suspensions from freshly collected specimens of HCC tumor, along with paired peritumor tissues and peripheral blood mononuclear cells (PBMCs) from 14 patients, we flow-cytometrically identified and quantified the relative frequencies of lymphocyte subsets within the tissues of origin. We found that the recruitment in the tumor of cytotoxic cells, namely the terminally differentiated CD4+ and CD8+ T cells (TEFF), is impaired, whereas the effector memory CD4+ T cells (TEM) are more attracted in this site. Concerning the other subsets, the frequency of NK CD56hi and NKT CD56hi cells infiltration in the tumor is increased, whereas that of NKT CD56low is reduced. Although CD4+ and CD8+ T cells settled in the tumor show a higher degree of activation than the circulating counterpart, they occur with a more exhausted phenotype. Overall, these data demonstrate the prevalently immunosuppressive nature of HCC microenvironment, and prompt us to search for strategies to enhance the activity of anti-tumor immune cell subsets.
Keywords: HCC; T cell response; antitumor immunity; immunotherapy.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

