Conducting Polymer Mediated Electrical Stimulation Induces Multilineage Differentiation with Robust Neuronal Fate Determination of Human Induced Pluripotent Stem Cells
- PMID: 32182797
- PMCID: PMC7140718
- DOI: 10.3390/cells9030658
Conducting Polymer Mediated Electrical Stimulation Induces Multilineage Differentiation with Robust Neuronal Fate Determination of Human Induced Pluripotent Stem Cells
Abstract
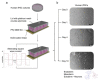
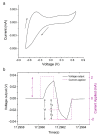
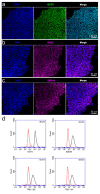
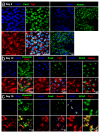
Electrical stimulation is increasingly being used to modulate human cell behaviour for biotechnological research and therapeutics. Electrically conductive polymers (CPs) such as polypyrrole (PPy) are amenable to in vitro and in vivo cell stimulation, being easy to synthesise with different counter ions (dopants) to augment biocompatibility and cell-effects. Extending our earlier work, which showed that CP-mediated electrical stimulation promotes human neural stem cell differentiation, here we report using electroactive PPy containing the anionic dopant dodecylbenzenesulfonate (DBS) to modulate the fate determination of human induced pluripotent stem cells (iPSCs). Remarkably, the stimulation without conventional chemical inducers resulted in the iPSCs differentiating to cells of the three germ lineages-endoderm, ectoderm, and mesoderm. The unstimulated iPSC controls remained undifferentiated. Phenotypic characterisation further showed a robust induction to neuronal fate with electrical stimulation, again without customary chemical inducers. Our findings add to the growing body of evidence supporting the use of electrical stimulation to augment stem cell differentiation, more specifically, pluripotent stem cell differentiation, and especially neuronal induction. Moreover, we have shown the versatility of electroactive PPy as a cell-compatible platform for advanced stem cell research and translation, including identifying novel mechanisms of fate regulation, tissue development, electroceuticals, and regenerative medicine.
Keywords: conductive polymers; differentiation; ectodermal; electrical stimulation; endodermal; induced pluripotent stem cells; mesodermal; neuronal; polypyrrole.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results”.
Figures

References
-
- Moffa A.H., Martin D., Alonzo A., Bennabi D., Blumberger D.M., Bensenor I.M., Daskalakis Z., Fregni F., Haffen E., Lisanby S.H., et al. Efficacy and acceptability of transcranial direct current stimulation (tDCS) for major depressive disorder: An individual patient data meta-analysis. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2019 doi: 10.1016/j.pnpbp.2019.109836. - DOI - PubMed
-
- Tomaskovic-Crook E., Zhang P., Ahtiainen A., Kaisvuo H., Lee C.Y., Beirne S., Aqrawe Z., Svirskis D., Hyttinen J., Wallace G.G., et al. Human Neural Tissues from Neural Stem Cells Using Conductive Biogel and Printed Polymer Microelectrode Arrays for 3D Electrical Stimulation. Adv. Healthc. Mater. 2019;8:e1900425. doi: 10.1002/adhm.201900425. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

