Oral Administration of Alpha Linoleic Acid Rescues Aβ-Induced Glia-Mediated Neuroinflammation and Cognitive Dysfunction in C57BL/6N Mice
- PMID: 32182943
- PMCID: PMC7140708
- DOI: 10.3390/cells9030667
Oral Administration of Alpha Linoleic Acid Rescues Aβ-Induced Glia-Mediated Neuroinflammation and Cognitive Dysfunction in C57BL/6N Mice
Abstract
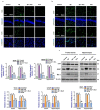
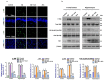
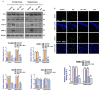
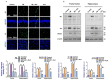
In this work, we evaluated the effects of alpha linoleic acid (ALA), an omega-3 polyunsaturated fatty acid, on amyloid-beta-induced glial-cell-mediated neuroinflammation, amyloidogenesis, and cognitive dysfunction in mice. After an infusion of Aβ1-42 (Aβ1-42, 5 μL/5 min/mouse, intracerebroventricular injection (i.c.v), and respective treatments of ALA (60 mg/kg per oral for six weeks), neuroinflammation, apoptotic markers, and synaptic markers were evaluated by Western blot and immunofluorescence analyses. According to our findings, the infusion of Aβ1-42 activated Toll-like receptor 4 (TLR4), glial fibrillary acidic protein (GFAP), and ionized calcium adaptor molecule 1 (Iba-1) in the frontal cortices and hippocampi of the Aβ1-42-injected mice to a greater extent than the Aβ1-42 + ALA-cotreated mice. Similarly, there was an elevated expression of phospho-c-Jun-N-terminal kinase (p-JNK), phospho-nuclear factor-kB p65 (p-NF-kB p65 (Ser536)), and tissue necrosis factor (TNF) in the Aβ1-42 infused mouse brains; interestingly, these markers were significantly reduced in the Aβ + ALA-cotreated group. The elevated expression of pro-apoptotic markers was observed during apoptotic cell death in the Aβ1-42-treated mouse brains, whereas these markers were markedly reduced in the Aβ + ALA-cotreated group. Moreover, Aβ1-42 infusion significantly increased amyloidogenesis, as assessed by the enhanced expression of the amyloid precursor proteins (APP) beta-amyloid cleaving enzyme-1 (BACE-1) and amyloid-beta (Aβ1-42) in the mouse brains, whereas these proteins were markedly reduced in the Aβ + ALA-cotreated group. We also checked the effects of ALA against Aβ-triggered synaptic dysfunction and memory dysfunction, showing that ALA significantly improved memory and synaptic functions in Aβ-treated mouse brains. These results indicated that ALA could be an applicable intervention in neuroinflammation, apoptotic cell loss, amyloidogenesis, and memory dysfunction via the inhibition of TLR4 and its downstream targets in Aβ + ALA-cotreated mouse brains.
Keywords: Alzheimer’s disease; neurodegeneration; neuroinflammation; omega-3 fatty acids.
Conflict of interest statement
The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures
References
-
- Ali M., Muhammad S., Shah M.R., Khan A., Rashid U., Farooq U., Ullah F., Sadiq A., Ayaz M., Ali M., et al. Neurologically Potent Molecules from Crataegus oxyacantha; Isolation, Anticholinesterase Inhibition, and Molecular Docking. Front. Pharmacol. 2017;8:327. doi: 10.3389/fphar.2017.00327. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

