Tuning the Polymorphism of the Anti-VEGF G-rich V7t1 Aptamer by Covalent Dimeric Constructs
- PMID: 32183039
- PMCID: PMC7139925
- DOI: 10.3390/ijms21061963
Tuning the Polymorphism of the Anti-VEGF G-rich V7t1 Aptamer by Covalent Dimeric Constructs
Abstract
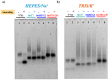
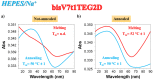
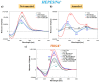
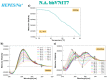
In the optimization process of nucleic acid aptamers, increased affinity and/or activity are generally searched by exploring structural analogues of the lead compound. In many cases, promising results have been obtained by dimerization of the starting aptamer. Here we studied a focused set of covalent dimers of the G-quadruplex (G4) forming anti-Vascular Endothelial Growth Factor (VEGF) V7t1 aptamer with the aim of identifying derivatives with improved properties. In the design of these covalent dimers, connecting linkers of different chemical nature, maintaining the same polarity along the strand or inverting it, have been introduced. These dimeric aptamers have been investigated using several biophysical techniques to disclose the conformational behavior, molecularity and thermal stability of the structures formed in different buffers. This in-depth biophysical characterization revealed the formation of stable G4 structures, however in some cases accompanied by alternative tridimensional arrangements. When tested for their VEGF165 binding and antiproliferative activity in comparison with V7t1, these covalent dimers showed slightly lower binding ability to the target protein but similar if not slightly higher antiproliferative activity on human breast adenocarcinoma MCF-7 cells. These results provide useful information for the design of improved dimeric aptamers based on further optimization of the linker joining the two consecutive V7t1 sequences.
Keywords: G-quadruplexes; V7t1; VEGF165; aptamers; biophysical characterization; covalent dimers.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Dimeric and Multimeric DNA Aptamers for Highly Effective Protein Recognition.Molecules. 2020 Nov 10;25(22):5227. doi: 10.3390/molecules25225227. Molecules. 2020. PMID: 33182593 Free PMC article. Review.
-
Insights into the G-rich VEGF-binding aptamer V7t1: when two G-quadruplexes are better than one!Nucleic Acids Res. 2019 Sep 5;47(15):8318-8331. doi: 10.1093/nar/gkz589. Nucleic Acids Res. 2019. PMID: 31276595 Free PMC article.
-
Selective light-up of dimeric G-quadruplex forming aptamers for efficient VEGF165 detection.Int J Biol Macromol. 2023 Jan 1;224:344-357. doi: 10.1016/j.ijbiomac.2022.10.128. Epub 2022 Oct 19. Int J Biol Macromol. 2023. PMID: 36270405
-
An Ensemble Docking Approach for Analyzing and Designing Aptamer Heterodimers Targeting VEGF165.Int J Mol Sci. 2024 Apr 5;25(7):4066. doi: 10.3390/ijms25074066. Int J Mol Sci. 2024. PMID: 38612876 Free PMC article.
-
G4 Aptamers: Trends in Structural Design.Mini Rev Med Chem. 2016;16(16):1321-1329. doi: 10.2174/1389557516666160321114715. Mini Rev Med Chem. 2016. PMID: 26996618 Review.
Cited by
-
Dimeric and Multimeric DNA Aptamers for Highly Effective Protein Recognition.Molecules. 2020 Nov 10;25(22):5227. doi: 10.3390/molecules25225227. Molecules. 2020. PMID: 33182593 Free PMC article. Review.
-
Fighting the Huntington's Disease with a G-Quadruplex-Forming Aptamer Specifically Binding to Mutant Huntingtin Protein: Biophysical Characterization, In Vitro and In Vivo Studies.Int J Mol Sci. 2022 Apr 27;23(9):4804. doi: 10.3390/ijms23094804. Int J Mol Sci. 2022. PMID: 35563194 Free PMC article.
-
Simultaneous Detection of VEGF and CEA by Time-Resolved Chemiluminescence Enzyme-Linked Aptamer Assay.Int J Nanomedicine. 2020 Dec 14;15:9975-9985. doi: 10.2147/IJN.S286317. eCollection 2020. Int J Nanomedicine. 2020. PMID: 33363367 Free PMC article.
-
Truncated Analogues of a G-Quadruplex-Forming Aptamer Targeting Mutant Huntingtin: Shorter Is Better!Int J Mol Sci. 2022 Oct 17;23(20):12412. doi: 10.3390/ijms232012412. Int J Mol Sci. 2022. PMID: 36293267 Free PMC article.
-
A terminal functionalization strategy reveals unusual binding abilities of anti-thrombin anticoagulant aptamers.Mol Ther Nucleic Acids. 2022 Nov 15;30:585-594. doi: 10.1016/j.omtn.2022.11.007. eCollection 2022 Dec 13. Mol Ther Nucleic Acids. 2022. PMID: 36457701 Free PMC article.
References
-
- Guo P., Fang Q., Tao H.Q., Schafer C.A., Fenton B.M., Ding I., Hu B., Cheng S.Y. Overexpression of vascular endothelial growth factor by MCF-7 breast cancer cells promotes estrogen-independent tumor growth in vivo. Cancer Res. 2003;63:4684–4691. - PubMed
-
- Siveen K.S., Prabhu K., Krishnankutty R., Kuttikrishnan S., Tsakou M., Alali F.Q., Dermime S., Mohammad R.M., Uddin S. Vascular endothelial growth factor (VEGF) signaling in tumour vascularization: Potential and challenges. Curr. Vasc. Pharmacol. 2017;15:339–351. doi: 10.2174/1570161115666170105124038. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

