Chitosan Oligosaccharides Attenuate Amyloid Formation of hIAPP and Protect Pancreatic β-Cells from Cytotoxicity
- PMID: 32183067
- PMCID: PMC7145300
- DOI: 10.3390/molecules25061314
Chitosan Oligosaccharides Attenuate Amyloid Formation of hIAPP and Protect Pancreatic β-Cells from Cytotoxicity
Abstract
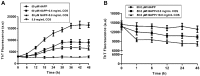
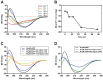
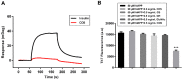
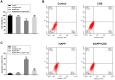
The deposition of aggregated human islet amyloid polypeptide (hIAPP) in the pancreas, that has been associated with β-cell dysfunction, is one of the common pathological features of patients with type 2 diabetes (T2D). Therefore, hIAPP aggregation inhibitors hold a promising therapeutic schedule for T2D. Chitosan oligosaccharides (COS) have been reported to exhibit a potential antidiabetic effect, but the function of COS on hIAPP amyloid formation remains elusive. Here, we show that COS inhibited the aggregation of hIAPP and disassembled preformed hIAPP fibrils in a dose-dependent manner by thioflavin T fluorescence assay, circular dichroism spectroscopy, and transmission electron microscope. Furthermore, COS protected mouse β-cells from cytotoxicity of amyloidogenic hIAPP, as well as apoptosis and cycle arrest. There was no direct binding of COS and hIAPP, as revealed by surface plasmon resonance analysis. In addition, both chitin-oligosaccharide and the acetylated monosaccharide of COS and glucosamine had no inhibition effect on hIAPP amyloid formation. It is presumed that, mechanistically, COS regulate hIAPP amyloid formation relating to the positive charge and degree of polymerization. These findings highlight the potential role of COS as inhibitors of hIAPP amyloid formation and provide a new insight into the mechanism of COS against diabetes.
Keywords: amyloid; chitosan oligosaccharides; cytotoxicity; human islet amyloid polypeptide; type 2 diabetes.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Inhibition of hIAPP Amyloid Aggregation and Pancreatic β-Cell Toxicity by OH-Terminated PAMAM Dendrimer.Small. 2016 Mar 23;12(12):1615-26. doi: 10.1002/smll.201502317. Epub 2016 Jan 25. Small. 2016. PMID: 26808649
-
Chitosan Oligosaccharides Inhibit/Disaggregate Fibrils and Attenuate Amyloid β-Mediated Neurotoxicity.Int J Mol Sci. 2015 May 8;16(5):10526-36. doi: 10.3390/ijms160510526. Int J Mol Sci. 2015. PMID: 26006224 Free PMC article.
-
Common fibrillar spines of amyloid-β and human islet amyloid polypeptide revealed by microelectron diffraction and structure-based inhibitors.J Biol Chem. 2018 Feb 23;293(8):2888-2902. doi: 10.1074/jbc.M117.806109. Epub 2017 Dec 27. J Biol Chem. 2018. PMID: 29282295 Free PMC article.
-
Human islet amyloid polypeptide (hIAPP) - a curse in type II diabetes mellitus: insights from structure and toxicity studies.Biol Chem. 2020 Sep 4;402(2):133-153. doi: 10.1515/hsz-2020-0174. Print 2021 Jan 27. Biol Chem. 2020. PMID: 33544470 Review.
-
An investigation into the potential action of polyphenols against human Islet Amyloid Polypeptide aggregation in type 2 diabetes.Int J Biol Macromol. 2023 Jan 15;225:318-350. doi: 10.1016/j.ijbiomac.2022.11.038. Epub 2022 Nov 15. Int J Biol Macromol. 2023. PMID: 36400215 Review.
Cited by
-
Chitosan Oligosaccharide Prevents Afatinib-Induced Barrier Disruption and Chloride Secretion through Modulation of AMPK, PI3K/AKT, and ERK Signaling in T84 Cells.Polymers (Basel). 2022 Oct 11;14(20):4255. doi: 10.3390/polym14204255. Polymers (Basel). 2022. PMID: 36297833 Free PMC article.
-
Chitosan Oligosaccharide Promotes Junction Barrier through Modulation of PI3K/AKT and ERK Signaling Intricate Interplay in T84 Cells.Polymers (Basel). 2023 Mar 28;15(7):1681. doi: 10.3390/polym15071681. Polymers (Basel). 2023. PMID: 37050295 Free PMC article.
-
Pharmacological inhibitors of β-cell dysfunction and death as therapeutics for diabetes.Front Endocrinol (Lausanne). 2023 Mar 15;14:1076343. doi: 10.3389/fendo.2023.1076343. eCollection 2023. Front Endocrinol (Lausanne). 2023. PMID: 37008937 Free PMC article. Review.
-
A new strategy to reconcile amyloid cross-seeding and amyloid prevention in a binary system of α-synuclein fragmental peptide and hIAPP.Protein Sci. 2022 Feb;31(2):485-497. doi: 10.1002/pro.4247. Epub 2021 Dec 8. Protein Sci. 2022. PMID: 34850985 Free PMC article.
-
The Ability of Some Polysaccharides to Disaggregate Lysozyme Amyloid Fibrils and Renature the Protein.Pharmaceutics. 2023 Feb 13;15(2):624. doi: 10.3390/pharmaceutics15020624. Pharmaceutics. 2023. PMID: 36839946 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

