Improvement of Testicular Steroidogenesis Using Flavonoids and Isoflavonoids for Prevention of Late-Onset Male Hypogonadism
- PMID: 32183155
- PMCID: PMC7139932
- DOI: 10.3390/antiox9030237
Improvement of Testicular Steroidogenesis Using Flavonoids and Isoflavonoids for Prevention of Late-Onset Male Hypogonadism
Abstract
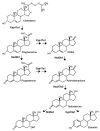
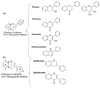
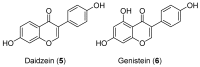
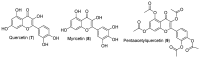
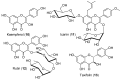
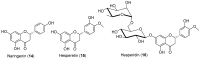
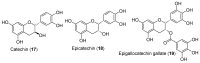
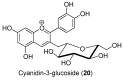
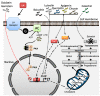
Androgen production, being important for male fertility, is mainly accomplished by the Leydig cells from the interstitial compartment of the testis. Testosterone plays a critical role in testis development, normal masculinization, and the maintenance of spermatogenesis. Within seminiferous tubules, appropriate Sertoli cell function is highly dependent on testicular androgen levels and is essential to initiate and maintain spermatogenesis. During aging, testosterone production by the testicular Leydig cells declines from the 30s in humans at a rate of 1% per year. This review outlines the recent findings regarding the use of flavonoids and isoflavonoids to improve testosterone production, contributing to normal spermatogenesis and preventing age-related degenerative diseases associated with testosterone deficiency. With the cumulation of information on the actions of different flavonoids and isoflavonoids on steroidogenesis in Leydig cells, we can now draw conclusions regarding the structure-activity relationship on androgen production. Indeed, flavonoids having a 5,7-dihydroxychromen-4-one backbone tend to increase the expression of the steroidogenic acute regulatory protein (StAR), being critical for the entry of cholesterol into the mitochondria, leading to increased testosterone production from testis Leydig cells. Therefore, flavonoids and isoflavonoids such as chrysin, apigenin, luteolin, quercetin, and daidzein may be effective in delaying the initiation of late-onset hypogonadism associated with aging in males.
Keywords: Leydig cells; androgen; flavonoids; polyphenols; testis; testosterone.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
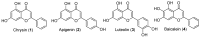
Comment in
-
Male and Female Sexual Function and Dysfunction; Andrology.J Urol. 2021 Apr;205(4):1208-1210. doi: 10.1097/JU.0000000000001618. Epub 2021 Jan 19. J Urol. 2021. PMID: 33464933 No abstract available.
References
-
- Camacho E.M., Huhtaniemi I.T., O’Neill T.W., Finn J.D., Pye S.R., Lee D.M., Tajar A., Bartfai G., Boonen S., Casanueva F.F., et al. Age-associated changes in hypothalamic-pituitary-testicular function in middle-aged and older men are modified by weight change and lifestyle factors: Longitudinal results from the European Male Ageing Study. Eur. J. Endocrinol. 2013;168:445–455. doi: 10.1530/EJE-12-0890. - DOI - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

