Ivermectin Inhibits Bovine Herpesvirus 1 DNA Polymerase Nuclear Import and Interferes with Viral Replication
- PMID: 32183205
- PMCID: PMC7143239
- DOI: 10.3390/microorganisms8030409
Ivermectin Inhibits Bovine Herpesvirus 1 DNA Polymerase Nuclear Import and Interferes with Viral Replication
Abstract
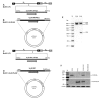
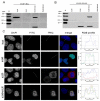
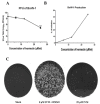
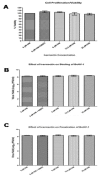
Bovine herpesvirus1 (BoHV-1) is a major bovine pathogen. Despite several vaccines being available to prevent viral infection, outbreaks are frequent and cause important economic consequences worldwide. The development of new antiviral drugs is therefore highly desirable. In this context, viral genome replication represents a potential target for therapeutic intervention. BoHV-1 genome is a dsDNA molecule whose replication takes place in the nuclei of infected cells and is mediated by a viral encoded DNA polymerase holoenzyme. Here, we studied the physical interaction and subcellular localization of BoHV-1 DNA polymerase subunits in cells for the first time. By means of co-immunoprecipitation and confocal laser scanning microscopy (CLSM) experiments, we could show that the processivity factor of the DNA polymerase pUL42 is capable of being autonomously transported into the nucleus, whereas the catalytic subunit pUL30 is not. Accordingly, a putative classic NLS (cNLS) was identified on pUL42 but not on pUL30. Importantly, both proteins could interact in the absence of other viral proteins and their co-expression resulted in accumulation of UL30 to the cell nucleus. Treatment of cells with Ivermectin, an anti-parasitic drug which has been recently identified as an inhibitor of importin α/β-dependent nuclear transport, reduced UL42 nuclear import and specifically reduced BoHV-1 replication in a dose-dependent manner, while virus attachment and entry into cells were not affected. Therefore, this study provides a new option of antiviral therapy for BoHV-1 infection with Ivermectin.
Keywords: BoHV-1; DNA polymerase holoenzyme; Ivermectin; NLS; antiviral; nucleocytoplasmic shuttling; pUL30; pUL42.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Workman A., Zhu L., Keel B.N., Smith T.P.L., Jones C. The Wnt Signaling Pathway Is Differentially Expressed during the Bovine Herpesvirus 1 Latency-Reactivation Cycle: Evidence That Two Protein Kinases Associated with Neuronal Survival, Akt3 and BMPR2, Are Expressed at Higher Levels during Latency. J. Virol. 2018;92:e01937-17. doi: 10.1128/JVI.01937-17. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

