A Halogen Bonding Perspective on Iodothyronine Deiodinase Activity
- PMID: 32183289
- PMCID: PMC7144113
- DOI: 10.3390/molecules25061328
A Halogen Bonding Perspective on Iodothyronine Deiodinase Activity
Abstract
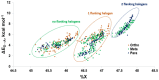
Iodothyronine deiodinases (Dios) are involved in the regioselective removal of iodine from thyroid hormones (THs). Deiodination is essential to maintain TH homeostasis, and disruption can have detrimental effects. Halogen bonding (XB) to the selenium of the selenocysteine (Sec) residue in the Dio active site has been proposed to contribute to the mechanism for iodine removal. Polybrominated diphenyl ethers (PBDEs) and polychlorinated biphenyls (PCBs) are known disruptors of various pathways of the endocrine system. Experimental evidence shows PBDEs and their hydroxylated metabolites (OH-BDEs) can inhibit Dio, while data regarding PCB inhibition are limited. These xenobiotics could inhibit Dio activity by competitively binding to the active site Sec through XB to prevent deiodination. XB interactions calculated using density functional theory (DFT) of THs, PBDEs, and PCBs to a methyl selenolate (MeSe-) arrange XB strengths in the order THs > PBDEs > PCBs in agreement with known XB trends. THs have the lowest energy C-X*-type unoccupied orbitals and overlap with the Se lp donor leads to high donor-acceptor energies and the greatest activation of the C-X bond. The higher energy C-Br* and C-Cl* orbitals similarly result in weaker donor-acceptor complexes and less activation of the C-X bond. Comparison of the I···Se interactions for the TH group suggest that a threshold XB strength may be required for dehalogenation. Only highly brominated PBDEs have binding energies in the same range as THs, suggesting that these compounds may inhibit Dio and undergo debromination. While these small models provide insight on the I···Se XB interaction itself, interactions with other active site residues are governed by regioselective preferences observed in Dios.
Keywords: endocrine disruption; halogen bonding; iodothyronine deiodinase; polybrominated diphenyl ethers (PBDEs); polychlorinated biphenyls (PCBs); thyroid hormones (THs); xenobiotics.
Conflict of interest statement
The authors declare no conflict of interest.
Figures







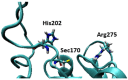


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

