Potential Role of Venular Amyloid in Alzheimer's Disease Pathogenesis
- PMID: 32183293
- PMCID: PMC7139584
- DOI: 10.3390/ijms21061985
Potential Role of Venular Amyloid in Alzheimer's Disease Pathogenesis
Abstract
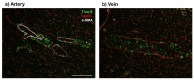
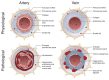
Insurmountable evidence has demonstrated a strong association between Alzheimer's disease (AD) and cerebral amyloid angiopathy (CAA), along with various other cerebrovascular diseases. One form of CAA, which is the accumulation of amyloid-beta peptides (Aβ) along cerebral vessel walls, impairs perivascular drainage pathways and contributes to cerebrovascular dysfunction in AD. To date, CAA research has been primarily focused on arterial Aβ, while the accumulation of Aβ in veins and venules were to a lesser extent. In this review, we describe preclinical models and clinical studies supporting the presence of venular amyloid and potential downstream pathological mechanisms that affect the cerebrovasculature in AD. Venous collagenosis, impaired cerebrovascular pulsatility, and enlarged perivascular spaces are exacerbated by venular amyloid and increase Aβ deposition, potentially through impaired perivascular clearance. Gaining a comprehensive understanding of the mechanisms involved in venular Aβ deposition and associated pathologies will give insight to how CAA contributes to AD and its association with AD-related cerebrovascular disease. Lastly, we suggest that special consideration should be made to develop Aβ-targeted therapeutics that remove vascular amyloid and address cerebrovascular dysfunction in AD.
Keywords: Alzheimer’s disease; Aβ; TgF344-AD rat model; cerebral amyloid angiopathy; perivascular clearance; vein/venule; venous collagenosis; venular amyloid.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Weller R.O., Subash M., Preston S.D., Mazanti I., Carare R.O. SYMPOSIUM: Clearance of Aβ from the Brain in Alzheimer′s Disease: Perivascular Drainage of Amyloid-β Peptides from the Brain and its Failure in Cerebral Amyloid Angiopathy and Alzheimer′s Disease. Brain Pathol. 2008;18:253–266. doi: 10.1111/j.1750-3639.2008.00133.x. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

