Aptamers Increase Biocompatibility and Reduce the Toxicity of Magnetic Nanoparticles Used in Biomedicine
- PMID: 32183370
- PMCID: PMC7148517
- DOI: 10.3390/biomedicines8030059
Aptamers Increase Biocompatibility and Reduce the Toxicity of Magnetic Nanoparticles Used in Biomedicine
Abstract
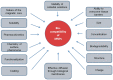
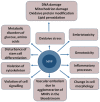
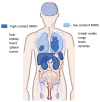
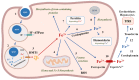
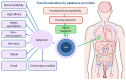
Aptamer-based approaches are very promising tools in nanomedicine. These small single-stranded DNA or RNA molecules are often used for the effective delivery and increasing biocompatibility of various therapeutic agents. Recently, magnetic nanoparticles (MNPs) have begun to be successfully applied in various fields of biomedicine. The use of MNPs is limited by their potential toxicity, which depends on their biocompatibility. The functionalization of MNPs by ligands increases biocompatibility by changing the charge and shape of MNPs, preventing opsonization, increasing the circulation time of MNPs in the blood, thus shielding iron ions and leading to the accumulation of MNPs only in the necessary organs. Among various ligands, aptamers, which are synthetic analogs of antibodies, turned out to be the most promising for the functionalization of MNPs. This review describes the factors that determine MNPs' biocompatibility and affect their circulation time in the bloodstream, biodistribution in organs and tissues, and biodegradation. The work also covers the role of the aptamers in increasing MNPs' biocompatibility and reducing toxicity.
Keywords: aptamers; biocompatibility; magnetic nanoparticles; toxicity.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

