Oxidative Stress and Mitochondrial Abnormalities Contribute to Decreased Endothelial Nitric Oxide Synthase Expression and Renal Disease Progression in Early Experimental Polycystic Kidney Disease
- PMID: 32183375
- PMCID: PMC7139316
- DOI: 10.3390/ijms21061994
Oxidative Stress and Mitochondrial Abnormalities Contribute to Decreased Endothelial Nitric Oxide Synthase Expression and Renal Disease Progression in Early Experimental Polycystic Kidney Disease
Abstract
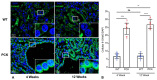
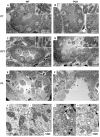
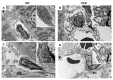
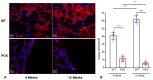
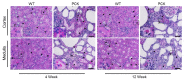
Vascular abnormalities are the most important non-cystic complications in Polycystic Kidney Disease (PKD) and contribute to renal disease progression. Endothelial dysfunction and oxidative stress are evident in patients with ADPKD, preserved renal function, and controlled hypertension. The underlying biological mechanisms remain unknown. We hypothesized that in early ADPKD, the reactive oxygen species (ROS)-producing nicotinamide adenine dinucleotide phosphate hydrogen (NAD(P)H)-oxidase complex-4 (NOX4), a major source of ROS in renal tubular epithelial cells (TECs) and endothelial cells (ECs), induces EC mitochondrial abnormalities, contributing to endothelial dysfunction, vascular abnormalities, and renal disease progression. Renal oxidative stress, mitochondrial morphology (electron microscopy), and NOX4 expression were assessed in 4- and 12-week-old PCK and Sprague-Dawley (wild-type, WT) control rats (n = 8 males and 8 females each). Endothelial function was assessed by renal expression of endothelial nitric oxide synthase (eNOS). Peritubular capillaries were counted in hematoxylin-eosin (H&E)-stained slides and correlated with the cystic index. The enlarged cystic kidneys of PCK rats exhibited significant accumulation of 8-hydroxyguanosine (8-OHdG) as early as 4 weeks of age, which became more pronounced at 12 weeks. Mitochondria of TECs lining cysts and ECs exhibited loss of cristae but remained preserved in non-cystic TECs. Renal expression of NOX4 was upregulated in TECs and ECs of PCK rats at 4 weeks of age and further increased at 12 weeks. Contrarily, eNOS immunoreactivity was lower in PCK vs. WT rats at 4 weeks and further decreased at 12 weeks. The peritubular capillary index was lower in PCK vs. WT rats at 12 weeks and correlated inversely with the cystic index. Early PKD is associated with NOX4-induced oxidative stress and mitochondrial abnormalities predominantly in ECs and TECs lining cysts. Endothelial dysfunction precedes capillary loss, and the latter correlates with worsening of renal disease. These observations position NOX4 and EC mitochondria as potential therapeutic targets in PKD.
Keywords: NOX4; endothelial dysfunction; mitochondria; oxidative stress; polycystic kidney disease.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Spithoven E.M., Kramer A., Meijer E., Orskov B., Wanner C., Caskey F., Collart F., Finne P., Fogarty D., Groothoff J.W., et al. Analysis of data from the ERA-EDTA Registry indicates that conventional treatments for chronic kidney disease do not reduce the need for renal replacement therapy in autosomal dominant polycystic kidney disease. Kidney Int. 2014;86:1244–1252. doi: 10.1038/ki.2014.120. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

