Polyvinyl Alcohol/Chitosan/Polyhexamethylene Biguanide Phase Separation System: A Potential Topical Antibacterial Formulation with Enhanced Antimicrobial Effect
- PMID: 32183411
- PMCID: PMC7146344
- DOI: 10.3390/molecules25061334
Polyvinyl Alcohol/Chitosan/Polyhexamethylene Biguanide Phase Separation System: A Potential Topical Antibacterial Formulation with Enhanced Antimicrobial Effect
Abstract
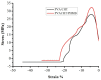
An aqueous polyvinyl alcohol (PVA)/chitosan (CHT)/polyhexamethylene biguanide (PHMB) blends (PVA/CHT/PHMB blends) has been developed as a potential low dose topical antibacterial formulation with enhanced antimicrobial effect. The preparation of PVA/CHT/PHMB blends was quite facilely, with just dissolved PVA, CHT, PHMB in water in order. There was the aggregates with 100 nm size around induced by phase separation in the blends and an aqueous two-phase system (ATPS) formed, as non-ionic polymer PVA formed a continuous phase and cationic polymer CHT and PHMB formed dispersed phases. The minimum inhibitory concentration (MIC) of PHMB in the PVA/CHT/PHMB blends was 0.5μg/mL, which was four times lower than the MIC of PHMB individually. A phase separation increased zeta potential mechanism was proposed to explain the enhanced antibacterial activities. In addition, the blends could easily form film on the skin surface with good water vapor permeability and be used as a liquid bandage to accelerate the scratch wound healing process of nude mouse. These findings provide experimental evidence that the PHMB-functionalized blends could be further explored as low-dose topical antibacterial formulations, and the nano-sized phase separation strategy could be used to design novel low-dose topical antibacterial formulations with an enhanced antimicrobial effect.
Keywords: chitosan; enhanced antimicrobial effect; low dose; phase separation; polyhexamethylene biguanide; polyvinyl alcohol; topical antibacterial formulation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures







References
-
- Spann C.T., Tutrone W.D., Weinberg J.M., Scheinfeld N., Ross B. Topical antibacterial agents for wound care: A primer. Dermatol. Surg. 2003;29:620–626. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

