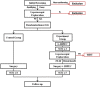
Study protocol of a multicenter phase III randomized controlled trial investigating the efficiency of the combination of neoadjuvant chemotherapy (NAC) and neoadjuvant laparoscopic intraperitoneal hyperthermic chemotherapy (NLHIPEC) followed by R0 gastrectomy with intraoperative HIPEC for advanced gastric cancer (AGC): dragon II trial
- PMID: 32183736
- PMCID: PMC7077163
- DOI: 10.1186/s12885-020-6701-2
Study protocol of a multicenter phase III randomized controlled trial investigating the efficiency of the combination of neoadjuvant chemotherapy (NAC) and neoadjuvant laparoscopic intraperitoneal hyperthermic chemotherapy (NLHIPEC) followed by R0 gastrectomy with intraoperative HIPEC for advanced gastric cancer (AGC): dragon II trial
Abstract
Background: Even though treatment modalities such as adjuvant systemic radio-chemotherapy and neoadjuvant chemotherapy (NAC) have individually have improved overall survival (OS) and progression-free survival (PFS) rates in advanced Gastric Cancer (AGC), the peritoneum still presides as a common site of treatment failure and disease recurrence. The role of hyperthermic intraperitoneal chemotherapy (HIPEC) has been acknowledged as prophylaxis for peritoneal carcinomatosis (PC) in AGC patients and in this study, we aim at investigating the safety and efficacy of the combination of neoadjuvant laparoscopic HIPEC (NLHIPEC) with NAC in the neoadjuvant phase followed by surgery of curative intent with intraoperative HIPEC followed by adjuvant chemotherapy (AC).
Methods: In this multicenter Phase III randomized controlled trial, 326 patients will be randomly separated into 2 groups into a 1:1 ratio after laparoscopic exploration. The experiment arm will receive the proposed comprehensive Dragon II regimen while the control group will undergo standard R0 D2 followed by 8 cycles of AC with oxaliplatin with S-1 (SOX) regimen. The Dragon II regimen comprises of 1 cycle of NLHIPEC for 60mins at 43 ± 0.5 °C with 80 mg/m2 of Paclitaxel followed by 3 cycles of NAC with SOX regimen and after assessment, standard R0 D2 gastrectomy with intraoperative HIPEC followed by 5 cycles of SOX regimen chemotherapy. The end-points for the study are 5 year PFS, 5 year OS, peritoneal metastasis rate (PMR) and morbidity rate.
Discussion: This study is one of the first to combine NLHIPEC with NAC in the preoperative phase which is speculated to provide local management of occult peritoneal carcinomatosis or peritoneal free cancer cells while NAC will promote tumor downsizing and down-staging. The addition of the intraoperative HIPEC is speculated to manage dissemination due to surgical trauma. Where the roles of intraoperative HIPEC and NAC have individually been investigated, this study provides innovative insight on a more comprehensive approach to management of AGC at high risk of peritoneal recurrence. It is expected that the combination of NLHIPEC with NAC and HIPEC will increase PFS by 15% and decrease PMR after gastrectomy of curative intent.
Trial registration: World Health Organization Clinical Trials - International Registry Platform (WHO-ICTRP) with Registration ID ChiCTR1900024552, Registered Prospectively on the 16th July, 2019.
Keywords: Advanced gastric Cancer; Intraperitoneal Hyperthermic chemotherapy; Neoadjuvant chemotherapy; Peritoneal Carcinomatosis; Progression-free survival.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
Similar articles
-
Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy versus palliative systemic chemotherapy in stomach cancer patients with peritoneal dissemination, the study protocol of a multicentre randomised controlled trial (PERISCOPE II).BMC Cancer. 2019 May 6;19(1):420. doi: 10.1186/s12885-019-5640-2. BMC Cancer. 2019. PMID: 31060544 Free PMC article.
-
Preventive HIPEC in combination with perioperative FLOT versus FLOT alone for resectable diffuse type gastric and gastroesophageal junction type II/III adenocarcinoma - the phase III "PREVENT"- (FLOT9) trial of the AIO /CAOGI /ACO.BMC Cancer. 2021 Oct 29;21(1):1158. doi: 10.1186/s12885-021-08872-8. BMC Cancer. 2021. PMID: 34715810 Free PMC article.
-
The protocol of a prospective, multicenter, randomized, controlled phase III study evaluating different cycles of oxaliplatin combined with S-1 (SOX) as neoadjuvant chemotherapy for patients with locally advanced gastric cancer: RESONANCE-II trial.BMC Cancer. 2021 Jan 5;21(1):20. doi: 10.1186/s12885-020-07764-7. BMC Cancer. 2021. PMID: 33402102 Free PMC article.
-
[Progress in prophylatic hyperthermic intraperitoneal chemotherapy for advanced gastric carcinoma].Zhonghua Wei Chang Wai Ke Za Zhi. 2018 May 25;21(5):593-599. Zhonghua Wei Chang Wai Ke Za Zhi. 2018. PMID: 29774943 Review. Chinese.
-
Neoadjuvant chemotherapy for gastric cancer. Is it a must or a fake?World J Gastroenterol. 2018 Jan 14;24(2):274-289. doi: 10.3748/wjg.v24.i2.274. World J Gastroenterol. 2018. PMID: 29375213 Free PMC article. Review.
Cited by
-
Locally advanced gastroesophageal junction cancer with pathological complete response to neoadjuvant therapy: a case report and literature review.Ann Transl Med. 2021 Mar;9(6):513. doi: 10.21037/atm-21-434. Ann Transl Med. 2021. PMID: 33850910 Free PMC article.
-
Praja2 suppresses the growth of gastric cancer by ubiquitylation of KSR1 and inhibiting MEK-ERK signal pathways.Aging (Albany NY). 2021 Jan 10;13(3):3886-3897. doi: 10.18632/aging.202356. Epub 2021 Jan 10. Aging (Albany NY). 2021. PMID: 33461174 Free PMC article.
-
Neoadjuvant intraperitoneal chemotherapy for advanced stage gastric cancer (Review).Exp Ther Med. 2021 Nov;22(5):1314. doi: 10.3892/etm.2021.10749. Epub 2021 Sep 17. Exp Ther Med. 2021. PMID: 34630668 Free PMC article. Review.
-
Clinical Effectiveness of Neoadjuvant Chemotherapy in Gastric Carcinoma and Exploration of Perioperative Imaging Assessment Parameters.Gastroenterol Res Pract. 2021 Apr 23;2021:5563136. doi: 10.1155/2021/5563136. eCollection 2021. Gastroenterol Res Pract. 2021. PMID: 33981339 Free PMC article.
-
Machine learning algorithm of ultrasound-mediated intestinal function recovery and nursing efficacy analysis of lower gastrointestinal malignant tumor after surgery.Pak J Med Sci. 2021;37(6):1662-1666. doi: 10.12669/pjms.37.6-WIT.4866. Pak J Med Sci. 2021. PMID: 34712302 Free PMC article.
References
-
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. - PubMed
-
- Cunningham D, Allum WH, Stenning SP, Thompson JN, Van de Velde CJ, Nicolson M, Scarffe JH, Lofts FJ, Falk SJ, Iveson TJ, et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med. 2006;355:11–20. - PubMed
-
- Ychou M, Boige V, Pignon JP, Conroy T, Bouché O, Lebreton G, Ducourtieux M, Bedenne L, Fabre JM, Saint-Aubert B, et al. Perioperative chemotherapy compared with surgery alone for resectable gastroesophageal adenocarcinoma: an FNCLCC and FFCD multicenter phase III trial. J Clin Oncol. 2011;29:1715–1721. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous