Neocortical tissue recovery in severe congenital obstructive hydrocephalus after intraventricular administration of bone marrow-derived mesenchymal stem cells
- PMID: 32183876
- PMCID: PMC7079418
- DOI: 10.1186/s13287-020-01626-6
Neocortical tissue recovery in severe congenital obstructive hydrocephalus after intraventricular administration of bone marrow-derived mesenchymal stem cells
Abstract
Background: In obstructive congenital hydrocephalus, cerebrospinal fluid accumulation is associated with high intracranial pressure and the presence of periventricular edema, ischemia/hypoxia, damage of the white matter, and glial reactions in the neocortex. The viability and short time effects of a therapy based on bone marrow-derived mesenchymal stem cells (BM-MSC) have been evaluated in such pathological conditions in the hyh mouse model.
Methods: BM-MSC obtained from mice expressing fluorescent mRFP1 protein were injected into the lateral ventricle of hydrocephalic hyh mice at the moment they present a very severe form of the disease. The effect of transplantation in the neocortex was compared with hydrocephalic hyh mice injected with the vehicle and non-hydrocephalic littermates. Neural cell populations and the possibility of transdifferentiation were analyzed. The possibility of a tissue recovering was investigated using 1H High-Resolution Magic Angle Spinning Nuclear Magnetic Resonance (1H HR-MAS NMR) spectroscopy, thus allowing the detection of metabolites/osmolytes related with hydrocephalus severity and outcome in the neocortex. An in vitro assay to simulate the periventricular astrocyte reaction conditions was performed using BM-MSC under high TNFα level condition. The secretome in the culture medium was analyzed in this assay.
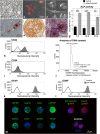
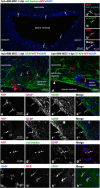
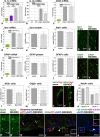
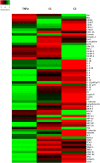
Results: Four days after transplantation, BM-MSC were found undifferentiated and scattered into the astrocyte reaction present in the damaged neocortex white matter. Tissue rejection to the integrated BM-MSC was not detected 4 days after transplantation. Hyh mice transplanted with BM-MSC showed a reduction in the apoptosis in the periventricular neocortex walls, suggesting a neuroprotector effect of the BM-MSC in these conditions. A decrease in the levels of metabolites/osmolytes in the neocortex, such as taurine and neuroexcytotoxic glutamate, also indicated a tissue recovering. Under high TNFα level condition in vitro, BM-MSC showed an upregulation of cytokine and protein secretion that may explain homing, immunomodulation, and vascular permeability, and therefore the tissue recovering.
Conclusions: BM-MSC treatment in severe congenital hydrocephalus is viable and leads to the recovery of the severe neurodegenerative conditions in the neocortex. NMR spectroscopy allows to follow-up the effects of stem cell therapy in hydrocephalus.
Keywords: Bone marrow-derived mesenchymal stem cells; Hydrocephalus; Reactive astrocytes; Spectroscopy.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


Similar articles
-
Identification of key molecular biomarkers involved in reactive and neurodegenerative processes present in inherited congenital hydrocephalus.Fluids Barriers CNS. 2021 Jul 2;18(1):30. doi: 10.1186/s12987-021-00263-2. Fluids Barriers CNS. 2021. PMID: 34215285 Free PMC article.
-
Generation of Periventricular Reactive Astrocytes Overexpressing Aquaporin 4 Is Stimulated by Mesenchymal Stem Cell Therapy.Int J Mol Sci. 2023 Mar 15;24(6):5640. doi: 10.3390/ijms24065640. Int J Mol Sci. 2023. PMID: 36982724 Free PMC article.
-
Comparison of Mesenchymal Stromal Cells Isolated from Murine Adipose Tissue and Bone Marrow in the Treatment of Spinal Cord Injury.Cell Transplant. 2018 Jul;27(7):1126-1139. doi: 10.1177/0963689718780309. Epub 2018 Jun 27. Cell Transplant. 2018. PMID: 29947256 Free PMC article.
-
Increased levels of tumour necrosis factor alpha (TNFα) but not transforming growth factor-beta 1 (TGFβ1) are associated with the severity of congenital hydrocephalus in the hyh mouse.Neuropathol Appl Neurobiol. 2014 Dec;40(7):911-32. doi: 10.1111/nan.12115. Neuropathol Appl Neurobiol. 2014. PMID: 24707814
-
Therapeutic efficiency of bone marrow-derived mesenchymal stem cells for liver fibrosis: A systematic review of in vivo studies.World J Gastroenterol. 2020 Dec 21;26(47):7444-7469. doi: 10.3748/wjg.v26.i47.7444. World J Gastroenterol. 2020. PMID: 33384547 Free PMC article.
Cited by
-
Neuroinflammatory pathways and potential therapeutic targets in neonatal post-hemorrhagic hydrocephalus.Pediatr Res. 2025 Mar;97(4):1345-1357. doi: 10.1038/s41390-024-03733-z. Epub 2024 Dec 26. Pediatr Res. 2025. PMID: 39725707 Review.
-
Human Umbilical Cord Mesenchymal Stem Cells Prevent Steroid-Induced Avascular Necrosis of the Femoral Head by Modulating Cellular Autophagy.Biomedicines. 2024 Dec 12;12(12):2817. doi: 10.3390/biomedicines12122817. Biomedicines. 2024. PMID: 39767723 Free PMC article.
-
Identification of key molecular biomarkers involved in reactive and neurodegenerative processes present in inherited congenital hydrocephalus.Fluids Barriers CNS. 2021 Jul 2;18(1):30. doi: 10.1186/s12987-021-00263-2. Fluids Barriers CNS. 2021. PMID: 34215285 Free PMC article.
-
Generation of Periventricular Reactive Astrocytes Overexpressing Aquaporin 4 Is Stimulated by Mesenchymal Stem Cell Therapy.Int J Mol Sci. 2023 Mar 15;24(6):5640. doi: 10.3390/ijms24065640. Int J Mol Sci. 2023. PMID: 36982724 Free PMC article.
-
Ventricular-subventricular zone stem cell niche adaptations in a mouse model of post-infectious hydrocephalus.Front Neurosci. 2024 Jul 31;18:1429829. doi: 10.3389/fnins.2024.1429829. eCollection 2024. Front Neurosci. 2024. PMID: 39145299 Free PMC article.
References
-
- Furey CG, Antwi P, Kahle KT. Congenital hydrocephalus. In: Limbrick DD, Leonard JR, editors. Cerebrospinal fluid disorders. Cham: Springer International Publishing; 2019. pp. 87–113.
-
- da Silva MC. Pathophysiology of hydrocephalus. In: Cinalli G, Sainte-Rose C, Maixner WJ, editors. Pediatric hydrocephalus. Milano: Springer Milan; 2005. pp. 65–77.

