Cerebrospinal fluid phospho-tau T217 outperforms T181 as a biomarker for the differential diagnosis of Alzheimer's disease and PET amyloid-positive patient identification
- PMID: 32183883
- PMCID: PMC7079453
- DOI: 10.1186/s13195-020-00596-4
Cerebrospinal fluid phospho-tau T217 outperforms T181 as a biomarker for the differential diagnosis of Alzheimer's disease and PET amyloid-positive patient identification
Abstract
Background: Cerebrospinal fluid biomarker profiles characterized by decreased amyloid-beta peptide levels and increased total and phosphorylated tau levels at threonine 181 (pT181) are currently used to discriminate between Alzheimer's disease and other neurodegenerative diseases. However, these changes are not entirely specific to Alzheimer's disease, and it is noteworthy that other phosphorylated isoforms of tau, possibly more specific for the disease process, have been described in the brain parenchyma of patients. The precise detection of these isoforms in biological fluids remains however a challenge.
Methods: In the present study, we used the latest quantitative mass spectrometry approach, which achieves a sensitive detection in cerebrospinal fluid biomarker of two phosphorylated tau isoforms, pT181 and pT217, and first analyzed a cohort of probable Alzheimer's disease patients and patients with other neurological disorders, including tauopathies, and a set of cognitively normal controls. We then checked the validity of our results on a second cohort comprising cognitively normal individuals and patients with mild cognitive impairments and AD stratified in terms of their amyloid status based on PiB-PET imaging methods.
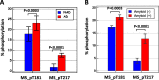
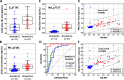
Results: In the first cohort, pT217 but not pT181 differentiated between Alzheimer's disease patients and those with other neurodegenerative diseases and control subjects much more specificity and sensitivity than pT181. T217 phosphorylation was increased by 6.0-fold in patients with Alzheimer's disease whereas T181 phosphorylation was only increased by 1.3-fold, when compared with control subjects. These results were confirmed in the case of a second cohort, in which the pT217 cerebrospinal fluid levels marked out amyloid-positive patients with a sensitivity and a specificity of more than 90% (AUC 0.961; CI 0.874 to 0.995). The pT217 concentrations were also highly correlated with the PiB-PET values (correlation coefficient 0.72; P < 0.001).
Conclusions: Increased cerebrospinal fluid pT217 levels, more than those of pT181, are highly specific biomarkers for detecting both the preclinical and advanced forms of Alzheimer's disease. This finding should greatly improve the diagnosis of Alzheimer's disease, along with the correlations found to exist between pT217 levels and PiB-PET data. It also suggests that pT217 is a promising potential target for therapeutic applications and that a link exists between amyloid and tau pathology.
Keywords: Alzheimer’s disease; Cerebrospinal fluid; Tau proteins.
Conflict of interest statement
Dr. Nicolas Barthélemy is a recipient of the Alzheimer’s Association Research Fellowship which supported this work. He declares that there are no conflicts of interest involved. Pr Sylvain Lehmann received institutional support from Montpellier University Hospital and the French National Research Agency for biomarker research. He received honoraria from Thermo Fisher, Roche, and Fujirebio for serving on scientific advisory boards. He is a shareholder at the Spot-to-Lab start-up company, which was not involved in this particular research. Dr. Audrey Gabelle received funds from the Fondation Philippe Chatrier. She declares that there are no conflicts of interest involved in this paper. Dr. Randall Bateman has received honoraria from Janssen, Pfizer, and Roche as a speaker; from Eisai as a consultant; and from Merck and Pfizer as an Advisory Board member. Washington University has submitted the US nonprovisional patent applications “Methods of Diagnosing and Treating Based on Site-Specific Tau Phosphorylation” (co-inventors RJB, NB) and “Central Nervous System Tau Kinetic Measurements as Diagnostic and Theragnostic Biomarkers” (co-inventors RJB, CS) and the provisional patent application “Quantification of Tau Isoforms, Modification, and Truncation for Assessment of Tauopathies” (co-inventors RJB, CS). Dr. Philippe Marin, François Becher, Christophe Hirtz, and Chihiro Sato (préciser Dr. ou Professor avant chaque nom) also declare that there are no competing interests.
Figures


References
-
- Association As 2018 Alzheimer’s disease facts and figures. Alzheimers Dement. 2018;14(3):367–429. doi: 10.1016/j.jalz.2018.02.001. - DOI
-
- Duits FH, Prins ND, Lemstra AW, Pijnenburg YA, Bouwman FH, Teunissen CE, et al. Diagnostic impact of CSF biomarkers for Alzheimer’s disease in a tertiary memory clinic. Alzheimer’s & dementia : the journal of the Alzheimer's Association. 2015;11(5):523–532. doi: 10.1016/j.jalz.2014.05.1753. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

