Drosophila enabled promotes synapse morphogenesis and regulates active zone form and function
- PMID: 32183907
- PMCID: PMC7076993
- DOI: 10.1186/s13064-020-00141-x
Drosophila enabled promotes synapse morphogenesis and regulates active zone form and function
Abstract
Background: Recent studies of synapse form and function highlight the importance of the actin cytoskeleton in regulating multiple aspects of morphogenesis, neurotransmission, and neural plasticity. The conserved actin-associated protein Enabled (Ena) is known to regulate development of the Drosophila larval neuromuscular junction through a postsynaptic mechanism. However, the functions and regulation of Ena within the presynaptic terminal has not been determined.
Methods: Here, we use a conditional genetic approach to address a presynaptic role for Ena on presynaptic morphology and ultrastructure, and also examine the pathway in which Ena functions through epistasis experiments.
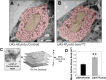
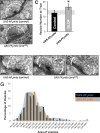
Results: We find that Ena is required to promote the morphogenesis of presynaptic boutons and branches, in contrast to its inhibitory role in muscle. Moreover, while postsynaptic Ena is regulated by microRNA-mediated mechanisms, presynaptic Ena relays the output of the highly conserved receptor protein tyrosine phosphatase Dlar and associated proteins including the heparan sulfate proteoglycan Syndecan, and the non-receptor Abelson tyrosine kinase to regulate addition of presynaptic varicosities. Interestingly, Ena also influences active zones, where it restricts active zone size, regulates the recruitment of synaptic vesicles, and controls the amplitude and frequency of spontaneous glutamate release.
Conclusion: We thus show that Ena, under control of the Dlar pathway, is required for presynaptic terminal morphogenesis and bouton addition and that Ena has active zone and neurotransmission phenotypes. Notably, in contrast to Dlar, Ena appears to integrate multiple pathways that regulate synapse form and function.
Keywords: Actin; Drosophila; Ena/VASP, Dlar; Receptor protein tyrosine phosphatase; Synapse.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures




Similar articles
-
Synapse development and maturation at the drosophila neuromuscular junction.Neural Dev. 2020 Aug 2;15(1):11. doi: 10.1186/s13064-020-00147-5. Neural Dev. 2020. PMID: 32741370 Free PMC article. Review.
-
Drosophila liprin-alpha and the receptor phosphatase Dlar control synapse morphogenesis.Neuron. 2002 Mar 28;34(1):27-38. doi: 10.1016/s0896-6273(02)00643-8. Neuron. 2002. PMID: 11931739
-
miR-8 controls synapse structure by repression of the actin regulator enabled.Development. 2014 May;141(9):1864-74. doi: 10.1242/dev.105791. Epub 2014 Apr 9. Development. 2014. PMID: 24718988 Free PMC article.
-
The HSPGs Syndecan and Dallylike bind the receptor phosphatase LAR and exert distinct effects on synaptic development.Neuron. 2006 Feb 16;49(4):517-31. doi: 10.1016/j.neuron.2006.01.026. Neuron. 2006. PMID: 16476662
-
Relevance of presynaptic actin dynamics for synapse function and mouse behavior.Exp Cell Res. 2015 Jul 15;335(2):165-71. doi: 10.1016/j.yexcr.2014.12.020. Epub 2015 Jan 8. Exp Cell Res. 2015. PMID: 25579398 Review.
Cited by
-
Synapse development and maturation at the drosophila neuromuscular junction.Neural Dev. 2020 Aug 2;15(1):11. doi: 10.1186/s13064-020-00147-5. Neural Dev. 2020. PMID: 32741370 Free PMC article. Review.
-
Molecular Logic of Synaptic Diversity Between Drosophila Tonic and Phasic Motoneurons.bioRxiv [Preprint]. 2023 Jan 19:2023.01.17.524447. doi: 10.1101/2023.01.17.524447. bioRxiv. 2023. Update in: Neuron. 2023 Nov 15;111(22):3554-3569.e7. doi: 10.1016/j.neuron.2023.07.019. PMID: 36711745 Free PMC article. Updated. Preprint.
-
Fe65: A Scaffolding Protein of Actin Regulators.Cells. 2021 Jun 25;10(7):1599. doi: 10.3390/cells10071599. Cells. 2021. PMID: 34202290 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

