Sulfamoyl Heteroarylcarboxylic Acids as Promising Metallo-β-Lactamase Inhibitors for Controlling Bacterial Carbapenem Resistance
- PMID: 32184250
- PMCID: PMC7078479
- DOI: 10.1128/mBio.03144-19
Sulfamoyl Heteroarylcarboxylic Acids as Promising Metallo-β-Lactamase Inhibitors for Controlling Bacterial Carbapenem Resistance
Abstract
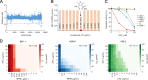
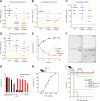
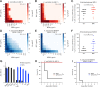
Production of metallo-β-lactamases (MBLs), which hydrolyze carbapenems, is a cause of carbapenem resistance in Enterobacteriaceae Development of effective inhibitors for MBLs is one approach to restore carbapenem efficacy in carbapenem-resistant Enterobacteriaceae (CRE). We report here that sulfamoyl heteroarylcarboxylic acids (SHCs) can competitively inhibit the globally spreading and clinically relevant MBLs (i.e., IMP-, NDM-, and VIM-type MBLs) at nanomolar to micromolar orders of magnitude. Addition of SHCs restored meropenem efficacy against 17/19 IMP-type and 7/14 NDM-type MBL-producing Enterobacteriaceae to satisfactory clinical levels. SHCs were also effective against IMP-type MBL-producing Acinetobacter spp. and engineered Escherichia coli strains overproducing individual minor MBLs (i.e., TMB-2, SPM-1, DIM-1, SIM-1, and KHM-1). However, SHCs were less effective against MBL-producing Pseudomonas aeruginosa Combination therapy with meropenem and SHCs successfully cured mice infected with IMP-1-producing E. coli and dually NDM-1/VIM-1-producing Klebsiella pneumoniae clinical isolates. X-ray crystallographic analyses revealed the inhibition mode of SHCs against MBLs; the sulfamoyl group of SHCs coordinated to two zinc ions, and the carboxylate group coordinated to one zinc ion and bound to positively charged amino acids Lys224/Arg228 conserved in MBLs. Preclinical testing revealed that the SHCs showed low toxicity in cell lines and mice and high stability in human liver microsomes. Our results indicate that SHCs are promising lead compounds for inhibitors of MBLs to combat MBL-producing CRE.IMPORTANCE Carbapenem antibiotics are the last resort for control of severe infectious diseases, bloodstream infections, and pneumonia caused by Gram-negative bacteria, including Enterobacteriaceae However, carbapenem-resistant Enterobacteriaceae (CRE) strains have spread globally and are a critical concern in clinical settings because CRE infections are recognized as a leading cause of increased mortality among hospitalized patients. Most CRE produce certain kinds of serine carbapenemases (e.g., KPC- and GES-type β-lactamases) or metallo-β-lactamases (MBLs), which can hydrolyze carbapenems. Although effective MBL inhibitors are expected to restore carbapenem efficacy against MBL-producing CRE, no MBL inhibitor is currently clinically available. Here, we synthesized 2,5-diethyl-1-methyl-4-sulfamoylpyrrole-3-carboxylic acid (SPC), which is a potent inhibitor of MBLs. SPC is a remarkable lead compound for clinically useful MBL inhibitors and can potentially provide a considerable benefit to patients receiving treatment for lethal infectious diseases caused by MBL-producing CRE.
Keywords: CRE; carbapenems; metallo-β-lactamase; sulfamoyl heteroarylcarboxylic acids.
Copyright © 2020 Wachino et al.
Figures


Similar articles
-
Hydroxyhexylitaconic acids as potent IMP-type metallo-β-lactamase inhibitors for controlling carbapenem resistance in Enterobacterales.Microbiol Spectr. 2024 Mar 5;12(3):e0234423. doi: 10.1128/spectrum.02344-23. Epub 2024 Feb 5. Microbiol Spectr. 2024. PMID: 38315122 Free PMC article.
-
Discovery of a Novel Metallo-β-Lactamase Inhibitor That Potentiates Meropenem Activity against Carbapenem-Resistant Enterobacteriaceae.Antimicrob Agents Chemother. 2018 Apr 26;62(5):e00074-18. doi: 10.1128/AAC.00074-18. Print 2018 May. Antimicrob Agents Chemother. 2018. PMID: 29530861 Free PMC article.
-
Structure-guided optimization of 1H-imidazole-2-carboxylic acid derivatives affording potent VIM-Type metallo-β-lactamase inhibitors.Eur J Med Chem. 2022 Jan 15;228:113965. doi: 10.1016/j.ejmech.2021.113965. Epub 2021 Nov 2. Eur J Med Chem. 2022. PMID: 34763944
-
Imipenem-Relebactam and Meropenem-Vaborbactam: Two Novel Carbapenem-β-Lactamase Inhibitor Combinations.Drugs. 2018 Jan;78(1):65-98. doi: 10.1007/s40265-017-0851-9. Drugs. 2018. PMID: 29230684 Review.
-
Cefepime-taniborbactam: Ushering in the era of metallo-β-lactamase inhibition.Pharmacotherapy. 2025 Jul;45(7):448-461. doi: 10.1002/phar.70036. Epub 2025 Jun 20. Pharmacotherapy. 2025. PMID: 40542539 Review.
Cited by
-
Ebola virus VP35 interacts non-covalently with ubiquitin chains to promote viral replication.PLoS Biol. 2024 Feb 29;22(2):e3002544. doi: 10.1371/journal.pbio.3002544. eCollection 2024 Feb. PLoS Biol. 2024. PMID: 38422166 Free PMC article.
-
Ebola Virus VP35 Interacts Non-Covalently with Ubiquitin Chains to Promote Viral Replication Creating New Therapeutic Opportunities.bioRxiv [Preprint]. 2023 Jul 15:2023.07.14.549057. doi: 10.1101/2023.07.14.549057. bioRxiv. 2023. Update in: PLoS Biol. 2024 Feb 29;22(2):e3002544. doi: 10.1371/journal.pbio.3002544. PMID: 37503276 Free PMC article. Updated. Preprint.
-
Structure, function, and evolution of metallo-β-lactamases from the B3 subgroup-emerging targets to combat antibiotic resistance.Front Chem. 2023 Jun 20;11:1196073. doi: 10.3389/fchem.2023.1196073. eCollection 2023. Front Chem. 2023. PMID: 37408556 Free PMC article. Review.
-
Recent Developments to Cope the Antibacterial Resistance via β-Lactamase Inhibition.Molecules. 2022 Jun 14;27(12):3832. doi: 10.3390/molecules27123832. Molecules. 2022. PMID: 35744953 Free PMC article. Review.
-
Structural Basis of Metallo-β-lactamase Inhibition by N-Sulfamoylpyrrole-2-carboxylates.ACS Infect Dis. 2021 Jun 11;7(6):1809-1817. doi: 10.1021/acsinfecdis.1c00104. Epub 2021 May 18. ACS Infect Dis. 2021. PMID: 34003651 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

