CLIC4 is a cytokinetic cleavage furrow protein that regulates cortical cytoskeleton stability during cell division
- PMID: 32184265
- PMCID: PMC7240295
- DOI: 10.1242/jcs.241117
CLIC4 is a cytokinetic cleavage furrow protein that regulates cortical cytoskeleton stability during cell division
Abstract
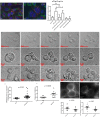
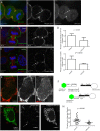
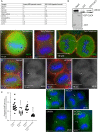
During mitotic cell division, the actomyosin cytoskeleton undergoes several dynamic changes that play key roles in progression through mitosis. Although the regulators of cytokinetic ring formation and contraction are well established, proteins that regulate cortical stability during anaphase and telophase have been understudied. Here, we describe a role for CLIC4 in regulating actin and actin regulators at the cortex and cytokinetic cleavage furrow during cytokinesis. We first describe CLIC4 as a new component of the cytokinetic cleavage furrow that is required for successful completion of mitotic cell division. We also demonstrate that CLIC4 regulates the remodeling of the sub-plasma-membrane actomyosin network within the furrow by recruiting MST4 kinase (also known as STK26) and regulating ezrin phosphorylation. This work identifies and characterizes new molecular players involved in regulating cortex stiffness and blebbing during the late stages of cytokinetic furrowing.
Keywords: Actin; Cell division; Cleavage furrow.
© 2020. Published by The Company of Biologists Ltd.
Conflict of interest statement
Competing interestsThe authors declare no competing or financial interests.
Figures





Similar articles
-
CLIC4 and CLIC1 bridge plasma membrane and cortical actin network for a successful cytokinesis.Life Sci Alliance. 2019 Dec 26;3(2):e201900558. doi: 10.26508/lsa.201900558. Print 2020 Feb. Life Sci Alliance. 2019. PMID: 31879279 Free PMC article.
-
Inhibition of polar actin assembly by astral microtubules is required for cytokinesis.Nat Commun. 2021 Apr 23;12(1):2409. doi: 10.1038/s41467-021-22677-0. Nat Commun. 2021. PMID: 33893302 Free PMC article.
-
Cleavage-furrow formation without F-actin in Chlamydomonas.Proc Natl Acad Sci U S A. 2020 Aug 4;117(31):18511-18520. doi: 10.1073/pnas.1920337117. Epub 2020 Jul 20. Proc Natl Acad Sci U S A. 2020. PMID: 32690698 Free PMC article.
-
Actin, microtubule, septin and ESCRT filament remodeling during late steps of cytokinesis.Curr Opin Cell Biol. 2018 Feb;50:27-34. doi: 10.1016/j.ceb.2018.01.007. Epub 2018 Feb 10. Curr Opin Cell Biol. 2018. PMID: 29438904 Review.
-
Centralspindlin in Rappaport's cleavage signaling.Semin Cell Dev Biol. 2016 May;53:45-56. doi: 10.1016/j.semcdb.2016.03.006. Epub 2016 Mar 7. Semin Cell Dev Biol. 2016. PMID: 26964770 Review.
Cited by
-
Diversity is the spice of life: An overview of how cytokinesis regulation varies with cell type.Front Cell Dev Biol. 2022 Nov 7;10:1007614. doi: 10.3389/fcell.2022.1007614. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 36420142 Free PMC article. Review.
-
The regulation of actin dynamics during cell division and malignancy.Am J Cancer Res. 2021 Sep 15;11(9):4050-4069. eCollection 2021. Am J Cancer Res. 2021. PMID: 34659876 Free PMC article. Review.
-
Induction of Pro-Fibrotic CLIC4 in Dermal Fibroblasts by TGF-β/Wnt3a Is Mediated by GLI2 Upregulation.Cells. 2022 Feb 3;11(3):530. doi: 10.3390/cells11030530. Cells. 2022. PMID: 35159339 Free PMC article.
-
Seeing is believing: tools to study the role of Rho GTPases during cytokinesis.Small GTPases. 2022 Jan;13(1):211-224. doi: 10.1080/21541248.2021.1957384. Epub 2021 Aug 18. Small GTPases. 2022. PMID: 34405757 Free PMC article. Review.
-
The Unusual Suspects in Cytokinesis: Fitting the Pieces Together.Front Cell Dev Biol. 2020 Jun 18;8:441. doi: 10.3389/fcell.2020.00441. eCollection 2020. Front Cell Dev Biol. 2020. PMID: 32626704 Free PMC article. Review.
References
-
- Argenzio E., Klarenbeek J., Kedziora K. M., Nahidiazar L., Isogai T., Perrakis A., Jalink K., Moolenaar W. H. and Innocenti M. (2018). Profilin binding couples chloride intracellular channel protein CLIC4 to RhoA–mDia2 signaling and filopodium formation. J. Biol. Chem. 293, 19161-19176. 10.1074/jbc.RA118.002779 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

