CLIC4 is a cytokinetic cleavage furrow protein that regulates cortical cytoskeleton stability during cell division
- PMID: 32184265
- PMCID: PMC7240295
- DOI: 10.1242/jcs.241117
CLIC4 is a cytokinetic cleavage furrow protein that regulates cortical cytoskeleton stability during cell division
Abstract
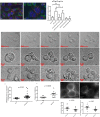
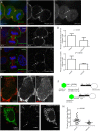
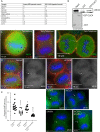
During mitotic cell division, the actomyosin cytoskeleton undergoes several dynamic changes that play key roles in progression through mitosis. Although the regulators of cytokinetic ring formation and contraction are well established, proteins that regulate cortical stability during anaphase and telophase have been understudied. Here, we describe a role for CLIC4 in regulating actin and actin regulators at the cortex and cytokinetic cleavage furrow during cytokinesis. We first describe CLIC4 as a new component of the cytokinetic cleavage furrow that is required for successful completion of mitotic cell division. We also demonstrate that CLIC4 regulates the remodeling of the sub-plasma-membrane actomyosin network within the furrow by recruiting MST4 kinase (also known as STK26) and regulating ezrin phosphorylation. This work identifies and characterizes new molecular players involved in regulating cortex stiffness and blebbing during the late stages of cytokinetic furrowing.
Keywords: Actin; Cell division; Cleavage furrow.
© 2020. Published by The Company of Biologists Ltd.
Conflict of interest statement
Competing interestsThe authors declare no competing or financial interests.
Figures





References
-
- Argenzio E., Klarenbeek J., Kedziora K. M., Nahidiazar L., Isogai T., Perrakis A., Jalink K., Moolenaar W. H. and Innocenti M. (2018). Profilin binding couples chloride intracellular channel protein CLIC4 to RhoA–mDia2 signaling and filopodium formation. J. Biol. Chem. 293, 19161-19176. 10.1074/jbc.RA118.002779 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

