Phase II double-blind randomised controlled trial of exogenous administration of melatonin in chronic pain (DREAM-CP): a study protocol
- PMID: 32184313
- PMCID: PMC7076250
- DOI: 10.1136/bmjopen-2019-034443
Phase II double-blind randomised controlled trial of exogenous administration of melatonin in chronic pain (DREAM-CP): a study protocol
Abstract
Introduction: Chronic pain is prevalent, and approximately half of patients with chronic pain experience sleep disturbance. Exogenous melatonin is licensed to treat primary insomnia and there is some evidence for analgesic effects of melatonin.The aim of this study is to investigate the effects of oral melatonin (as Circadin) 2 mg at night in adults with severe non-malignant pain of at least 3 months' duration.
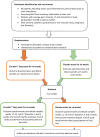
Methods and analysis: We will conduct a randomised double-blind placebo-controlled cross-over study. The primary outcome is sleep disturbance. Secondary outcomes are pain intensity, actigraphy, fatigue, reaction time testing, serum melatonin and endogenous opioid peptide levels along with patient views about study participation.We aim to recruit 60 patients with severe chronic pain (average pain intensity ≥7 on the Brief Pain Inventory (BPI)) from a tertiary referral pain clinic in Northeast Scotland. Participants will be randomised to receive melatonin (as modified release Circadin) 2 mg daily for 6 weeks or placebo, followed by a 4-week washout period, then 6 weeks treatment with the treatment they did not receive. Participants will complete the Verran Snyder-Halpern Sleep Scale, Pittsburgh Sleep Quality Index, Pain and Sleep Questionnaire 3-item index, BPI and psychomotor vigilance reaction time testing at 6 points over 20 weeks. Actigraphy watches will be used to provide objective measures of sleep duration and latency and other sleep measures and will prompt patients to report contemporaneous pain and fatigue scores daily.Cross-over analyses will include tests for effects of treatment, period, treatment-period interaction (carryover effect) and sequence. Within-patient effects and longitudinal data will be analysed using mixed linear models, accounting for potential confounders.
Ethics and dissemination: Approved by Office for Research Ethics Committees Northern Ireland, reference 19/NI/0007. Results will be published in peer-reviewed journals and will be presented at national and international conferences.
Trial registration number: ISRCTN12861060.
Keywords: pain management; sleep medicine.
© Author(s) (or their employer(s)) 2020. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
References
-
- Karaman S, Karaman T, Dogru S, et al. . Prevalence of sleep disturbance in chronic pain. Eur Rev Med Pharmacol Sci 2014;18:2475–81. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous