Sensory-Directed Genetic and Biochemical Characterization of Volatile Terpene Production in Kiwifruit
- PMID: 32184346
- PMCID: PMC7210626
- DOI: 10.1104/pp.20.00186
Sensory-Directed Genetic and Biochemical Characterization of Volatile Terpene Production in Kiwifruit
Abstract
Terpene volatiles are found in many important fruit crops, but their relationship to flavor is poorly understood. Here, we demonstrate using sensory descriptive and discriminant analysis that 1,8-cineole contributes a key floral/eucalyptus note to the aroma of ripe 'Hort16A' kiwifruit (Actinidia chinensis). Two quantitative trait loci (QTLs) for 1,8-cineole production were identified on linkage groups 27 and 29a in a segregating A. chinensis population, with the QTL on LG29a colocating with a complex cluster of putative terpene synthase (TPS)-encoding genes. Transient expression in Nicotiana benthamiana and analysis of recombinant proteins expressed in Escherichia coli showed four genes in the cluster (AcTPS1a-AcTPS1d) encoded functional TPS enzymes, which produced predominantly sabinene, 1,8-cineole, geraniol, and springene, respectively. The terpene profile produced by AcTPS1b closely resembled the terpenes detected in red-fleshed A chinensis AcTPS1b expression correlated with 1,8-cineole content in developing/ripening fruit and also showed a positive correlation with 1,8-cineole content in the mapping population, indicating the basis for segregation is an expression QTL. Transient overexpression of AcTPS1b in Actinidia eriantha fruit confirmed this gene produced 1,8-cineole in Actinidia Structure-function analysis showed AcTPS1a and AcTPS1b are natural variants at key TPS catalytic site residues previously shown to change enzyme specificity in vitro. Together, our results indicate that AcTPS1b is a key gene for production of the signature flavor terpene 1,8-cineole in ripe kiwifruit. Using a sensory-directed strategy for compound identification provides a rational approach for applying marker-aided selection to improving flavor in kiwifruit as well as other fruits.
© 2020 American Society of Plant Biologists. All Rights Reserved.
Figures




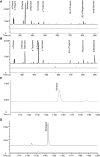

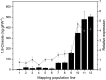
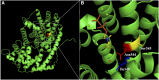
References
-
- Aharoni A, Jongsma M, Kim T-Y, Ri M-B, Giri A, Verstappen F, Schwab W, Bouwmeester H(2006) Metabolic engineering of terpenoid biosynthesis in plants. Phytochem Rev 5: 49–58
-
- Ares G, Jaeger SR(2015) Check-all-that-apply (CATA) questions with consumers in practice: Experimental considerations and impact on outcome In Delarue J, Lawlor JB, and Rogeaux M, eds, Rapid Sensory Profiling Techniques. Woodhead Publishing, Cambridge, pp 227–245
-
- Atkinson RG.(2018) Phenylpropenes: Occurrence, distribution, and biosynthesis in fruit. J Agric Food Chem 66: 2259–2272 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

