Inactivation of Non-Enveloped Viruses and Bacteria by an Electrically Charged Disinfectant Containing Meso-Structure Nanoparticles via Modification of the Genome
- PMID: 32184593
- PMCID: PMC7055524
- DOI: 10.2147/IJN.S229880
Inactivation of Non-Enveloped Viruses and Bacteria by an Electrically Charged Disinfectant Containing Meso-Structure Nanoparticles via Modification of the Genome
Abstract
Introduction: A previous study demonstrated the virucidal effect of an electrically charged disinfectant (CAC-717), which contains meso-structure nanoparticles, on enveloped viruses (influenza viruses). However, the effect of CAC-717 on other microorganisms and the mechanisms by which CAC-717 inactivates the microorganisms remain unclear. In this study, CAC-717 was further evaluated in terms of its biocidal and virucidal activity as well as its effect on bacterial and viral nucleic acids.
Methods: The inactivation effects of CAC-717 against various microorganisms [non-enveloped virus, feline calicivirus (FCV); bacteria, Salmonella enterica and Escherichia coli] were investigated by comparing the viral titer of the medium tissue culture infectious dose (TCID50) and the D value (estimated treatment time required to reduce the number of microorganisms by 90%). Furthermore, the effects of CAC-717 on viral and bacterial genomic RNA/DNA were examined using a polymerase chain reaction (PCR).
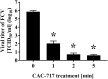
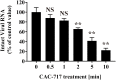
Results: Treatment of an equal volume of CAC-717 with cell lysate infected with a non-enveloped virus, feline calicivirus (FCV), reduced the TCID50. Viral titer dropped below the detection limit after 2 min of treatment. The D value of FCV was 0.256 min (average of multiple endpoint D values) and endpoint D value was 0.341 min. The D value for E. coli and S. enterica was 0.290 min and 0.080 min (average of multiple endpoint D values), respectively and the endpoint D value was 0.545 min and 0.054 min, respectively. In addition, PCR showed the inhibition of nucleic acid amplification of the RNA and DNA genome of FCV and bacteria, respectively.
Conclusion: Our findings suggest that CAC-717 inactivates viruses and bacteria by modifying the viral and bacterial nucleic acids.
Keywords: E. coli; Salmonella; class I disinfectant; feline calicivirus; food safety; meso-structure.
© 2020 Sakudo et al.
Conflict of interest statement
K.F. and R.O. are employed by the Mineral Activation Technical Research Center and Santa Mineral Co., Ltd., respectively. The authors declare that they have no other conflicts of interest with the content of this article.
Figures




Similar articles
-
A Review of CAC-717, a Disinfectant Containing Calcium Hydrogen Carbonate Mesoscopic Crystals.Microorganisms. 2025 Feb 25;13(3):507. doi: 10.3390/microorganisms13030507. Microorganisms. 2025. PMID: 40142400 Free PMC article. Review.
-
Inactivation of coxsackievirus B4, feline calicivirus and herpes simplex virus type 1: unexpected virucidal effect of a disinfectant on a non-enveloped virus applied onto a surface.Intervirology. 2013;56(4):224-30. doi: 10.1159/000350556. Epub 2013 Jun 13. Intervirology. 2013. PMID: 23774583
-
Comparison of chlorine and peroxyacetic-based disinfectant to inactivate Feline calicivirus, Murine norovirus and Hepatitis A virus on lettuce.Int J Food Microbiol. 2011 Nov 15;151(1):98-104. doi: 10.1016/j.ijfoodmicro.2011.08.011. Epub 2011 Aug 22. Int J Food Microbiol. 2011. PMID: 21924791
-
Key role of singlet oxygen and peroxynitrite in viral RNA damage during virucidal effect of plasma torch on feline calicivirus.Sci Rep. 2018 Dec 18;8(1):17947. doi: 10.1038/s41598-018-36779-1. Sci Rep. 2018. PMID: 30560882 Free PMC article.
-
The Influence of Simulated Organic Matter on the Inactivation of Viruses: A Review.Viruses. 2024 Jun 26;16(7):1026. doi: 10.3390/v16071026. Viruses. 2024. PMID: 39066189 Free PMC article. Review.
Cited by
-
Inactivation of Scrapie Prions by the Electrically Charged Disinfectant CAC-717.Pathogens. 2020 Jul 3;9(7):536. doi: 10.3390/pathogens9070536. Pathogens. 2020. PMID: 32635278 Free PMC article.
-
Universal Virucidal Activity of Calcium Bicarbonate Mesoscopic Crystals That Provides an Effective and Biosafe Disinfectant.Microorganisms. 2022 Jan 24;10(2):262. doi: 10.3390/microorganisms10020262. Microorganisms. 2022. PMID: 35208717 Free PMC article.
-
A Review of CAC-717, a Disinfectant Containing Calcium Hydrogen Carbonate Mesoscopic Crystals.Microorganisms. 2025 Feb 25;13(3):507. doi: 10.3390/microorganisms13030507. Microorganisms. 2025. PMID: 40142400 Free PMC article. Review.
-
Application of a roller conveyor type plasma disinfection device with fungus-contaminated citrus fruits.AMB Express. 2021 Jan 9;11(1):16. doi: 10.1186/s13568-020-01177-2. AMB Express. 2021. PMID: 33423150 Free PMC article.
-
Antibiotic-Resistant and Non-Resistant Bacteria Display Similar Susceptibility to Dielectric Barrier Discharge Plasma.Int J Mol Sci. 2020 Aug 31;21(17):6326. doi: 10.3390/ijms21176326. Int J Mol Sci. 2020. PMID: 32878289 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

