Updated Evaluation of Dupilumab in the Treatment of Asthma: Patient Selection and Reported Outcomes
- PMID: 32184610
- PMCID: PMC7061408
- DOI: 10.2147/TCRM.S192392
Updated Evaluation of Dupilumab in the Treatment of Asthma: Patient Selection and Reported Outcomes
Abstract
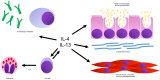
Uncontrolled asthma continues to be a problem for many patients with moderate-to-severe allergic asthma. Dupilumab, which blocks the receptors for interleukin-4 and interleukin-13, has been effective in reducing asthma exacerbations, improving forced expiratory volume in one second (FEV1), and reducing oral corticosteroid use. When selecting patients for dupilumab, it is important to consider entry criteria for the original studies, subgroups that have responded best, and the presence of comorbid diseases that may also respond to dupilumab. Factors that were considered when selecting patients likely to respond to dupilumab in asthma studies include: failure of moderate or high dose inhaled steroids in combination with an additional controller medication, baseline FEV1 reversibility of 12% or greater, and Asthma Control Questionnaire > 1.5. The baseline characteristics that predicted a better response to dupilumab included blood eosinophils > 150 cells/mm3 and fractional exhaled nitric oxide > 25 parts per billion. Comorbidities that may also respond to treatment with dupilumab include atopic dermatitis, chronic rhinosinusitis, and allergic rhinitis. A combination of these factors should be considered when selecting the patients most likely to benefit from dupilumab.
Keywords: allergic asthma; comorbidity; dupilumab; eosinophils; nitric oxide.
© 2020 Brooks.
Conflict of interest statement
The Asthma and Allergy Center, employer of Dr. Brooks, has received payment for the conduct of multiple clinical trials from Sanofi, AstraZeneca, GlaxoSmithKline, Novartis, and Teva. The author reports no other conflicts of interest in this work.
Figures
References
-
- Dupixent [package insert]. Tarrytown, NY: Regeneron Pharmaceuticals; 2019.
Publication types
LinkOut - more resources
Full Text Sources