Inhaled Methoxyflurane versus Intravenous Morphine for Severe Trauma Pain in the Emergency Setting: Subgroup Analysis of MEDITA, a Multicenter, Randomized, Controlled, Open-Label Trial
- PMID: 32184653
- PMCID: PMC7064290
- DOI: 10.2147/JPR.S240911
Inhaled Methoxyflurane versus Intravenous Morphine for Severe Trauma Pain in the Emergency Setting: Subgroup Analysis of MEDITA, a Multicenter, Randomized, Controlled, Open-Label Trial
Abstract
Purpose: Opioid analgesics remain the cornerstone of treatment for severe trauma pain in the emergency setting, but there are barriers to their use. This post hoc analysis of a previously reported trial (MEDITA) investigated the efficacy and safety of low-dose methoxyflurane versus intravenous (IV) morphine for severe trauma pain.
Patients and methods: MEDITA was a Phase IIIb, randomized, active-controlled, parallel-group, open-label study in Italian pre-hospital units and emergency departments (EudraCT: 2017-001565-25; NCT03585374). Adult patients (N=272) with moderate-to-severe trauma pain (score ≥4 on the Numerical Rating Scale [NRS]) were randomized 1:1 to inhaled methoxyflurane (3 mL) or standard analgesic treatment (SAT; IV paracetamol 1g or ketoprofen 100mg for moderate pain [NRS 4-6] and IV morphine 0.1mg/kg for severe pain [NRS ≥7]). Analyses were performed for the severe pain subgroup. The primary efficacy variable was the overall change from baseline in visual analog scale (VAS) pain intensity at 3, 5 and 10min post-randomization. Non-inferiority of methoxyflurane versus morphine was concluded if the upper 95% confidence interval (CI) for the treatment difference was <1; superiority was concluded if the upper 95% CI was <0.
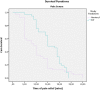
Results: Ninety-three patients (methoxyflurane: 49; SAT: 44) were included in the severe pain intention-to-treat population. The reduction in VAS pain intensity over the first 10min was superior for methoxyflurane versus morphine (adjusted mean treatment difference: -5.54mm; 95% CI: -10.49, -0.59mm; p=0.029). Median time to onset of pain relief was 9min for methoxyflurane and 15min for morphine. Patients rated treatment efficacy and physicians rated treatment practicality "Excellent" or "Very good" for more methoxyflurane-treated patients (42.8% and 67.3%) than morphine-treated patients (18.1% and 22.8%). Adverse events, all non-serious, were reported in 20.4% of methoxyflurane-treated patients and in 4.8% of morphine-treated patients.
Conclusion: Methoxyflurane provided superior short-term pain relief to IV morphine in patients with severe trauma pain and offers an effective non-narcotic treatment option.
Keywords: acute pain; analgesic; emergency department; methoxyflurane; morphine; prehospital.
© 2020 Voza et al.
Conflict of interest statement
Elisabetta Bonafede is an employee of the clinical research organization that conducted the study. Antonella Sblendido, Amedeo Soldi and Alberto Farina are employees of Mundipharma Pharmaceuticals srl. The authors report no other conflicts of interest in this work.
Figures




References
-
- World Health Organization. WHO’s cancer pain ladder for adults 1986. Available from: http://www.who.int/cancer/palliative/painladder/en/. Accessed January24, 2020.
LinkOut - more resources
Full Text Sources
Research Materials

