Evidence for the alloimmune basis and prognostic significance of Borderline T cell-mediated rejection
- PMID: 32185878
- PMCID: PMC7496654
- DOI: 10.1111/ajt.15860
Evidence for the alloimmune basis and prognostic significance of Borderline T cell-mediated rejection
Abstract
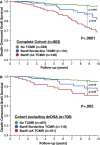
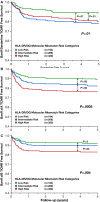
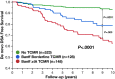
Prognostic biomarkers of T cell-mediated rejection (TCMR) have not been adequately studied in the modern era. We evaluated 803 renal transplant recipients and correlated HLA-DR/DQ molecular mismatch alloimmune risk categories (low, intermediate, high) with the severity, frequency, and persistence of TCMR. Allograft survival was reduced in recipients with Banff Borderline (hazard ratio [HR] 2.4, P = .003) and Banff ≥ IA TCMR (HR 4.3, P < .0001) including a subset who never developed de novo donor-specific antibodies (P = .002). HLA-DR/DQ molecular mismatch alloimmune risk categories were multivariate correlates of Banff Borderline and Banff ≥ IA TCMR and correlated with the severity and frequency of rejection episodes. Recipient age, HLA-DR/DQ molecular mismatch category, and cyclosporin vs tacrolimus immunosuppression were independent correlates of Banff Borderline and Banff ≥ IA TCMR. In the subset treated with tacrolimus (720/803) recipient age, HLA-DR/DQ molecular mismatch category, and tacrolimus coefficient of variation were independent correlates of TCMR. The correlation of HLA-DR/DQ molecular mismatch category with TCMR, including Borderline, provides evidence for their alloimmune basis. HLA-DR/DQ molecular mismatch may represent a precise prognostic biomarker that can be applied to tailor immunosuppression or design clinical trials based on individual patient risk.
Keywords: T cell-mediated rejection (TCMR); clinical research / practice; graft survival; histocompatibility; immunosuppression / immune modulation; kidney transplantation / nephrology; major histocompatibility complex (MHC); risk assessment / risk stratification.
© 2020 The Authors. American Journal of Transplantation published by Wiley Periodicals LLC on behalf of The American Society of Transplantation and the American Society of Transplant Surgeons.
Conflict of interest statement
The authors of this manuscript have conflicts to disclose as described by the
Figures
References
-
- U.S. Food and Drug Administration . Precision medicine. https://www.fda.gov/medical‐devices/vitro‐diagnostics/precision‐medicine. Accesed September 28, 2018.
-
- Dugast E, Soulillou J‐P, Foucher Y, et al. Failure of calcineurin inhibitor (Tacrolimus) weaning randomized trial in long‐term stable kidney transplant recipients. Am J Transplant. 2016;16(11):3255‐3261. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

