Tracking the cargo of extracellular symbionts into host tissues with correlated electron microscopy and nanoscale secondary ion mass spectrometry imaging
- PMID: 32185893
- PMCID: PMC7765731
- DOI: 10.1111/cmi.13177
Tracking the cargo of extracellular symbionts into host tissues with correlated electron microscopy and nanoscale secondary ion mass spectrometry imaging
Abstract
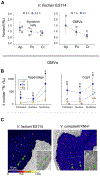
Extracellular bacterial symbionts communicate biochemically with their hosts to establish niches that foster the partnership. Using quantitative ion microprobe isotopic imaging (nanoscale secondary ion mass spectrometry [NanoSIMS]), we surveyed localization of 15 N-labelled molecules produced by the bacterium Vibrio fischeri within the cells of the symbiotic organ of its host, the Hawaiian bobtail squid, and compared that with either labelled non-specific species or amino acids. In all cases, two areas of the organ's epithelia were significantly more 15 N enriched: (a) surface ciliated cells, where environmental symbionts are recruited, and (b) the organ's crypts, where the symbiont population resides in the host. Label enrichment in all cases was strongest inside host cell nuclei, preferentially in the euchromatin regions and the nucleoli. This permissiveness demonstrated that uptake of biomolecules is a general mechanism of the epithelia, but the specific responses to V. fischeri cells recruited to the organ's surface are due to some property exclusive to this species. Similarly, in the organ's deeper crypts, the host responds to common bacterial products that only the specific symbiont can present in that location. The application of NanoSIMS allows the discovery of such distinct modes of downstream signalling dependent on location within the host and provides a unique opportunity to study the microbiogeographical patterns of symbiotic dialogue.
Keywords: 15N-labeled bacteria; host-microbe communication; squid-vibrio.
© 2020 John Wiley & Sons Ltd.
Conflict of interest statement
CONFLICT OF INTEREST
The authors declare no conflict of interest.
Figures


Similar articles
-
Interactions of Symbiotic Partners Drive the Development of a Complex Biogeography in the Squid-Vibrio Symbiosis.mBio. 2020 May 26;11(3):e00853-20. doi: 10.1128/mBio.00853-20. mBio. 2020. PMID: 32457244 Free PMC article.
-
The impact of Vibrio fischeri strain variation on host colonization.Curr Opin Microbiol. 2019 Aug;50:15-19. doi: 10.1016/j.mib.2019.09.002. Epub 2019 Oct 6. Curr Opin Microbiol. 2019. PMID: 31593868 Free PMC article. Review.
-
A Small Molecule Coordinates Symbiotic Behaviors in a Host Organ.mBio. 2021 Mar 9;12(2):e03637-20. doi: 10.1128/mBio.03637-20. mBio. 2021. PMID: 33688014 Free PMC article.
-
Characterization of the cell polarity gene crumbs during the early development and maintenance of the squid-vibrio light organ symbiosis.Dev Genes Evol. 2017 Nov;227(6):375-387. doi: 10.1007/s00427-017-0576-5. Epub 2017 Jan 20. Dev Genes Evol. 2017. PMID: 28105525 Free PMC article.
-
A lasting symbiosis: how Vibrio fischeri finds a squid partner and persists within its natural host.Nat Rev Microbiol. 2021 Oct;19(10):654-665. doi: 10.1038/s41579-021-00557-0. Epub 2021 Jun 4. Nat Rev Microbiol. 2021. PMID: 34089008 Free PMC article. Review.
Cited by
-
Getting the Message Out: the Many Modes of Host-Symbiont Communication during Early-Stage Establishment of the Squid-Vibrio Partnership.mSystems. 2021 Oct 26;6(5):e0086721. doi: 10.1128/mSystems.00867-21. Epub 2021 Sep 28. mSystems. 2021. PMID: 34581595 Free PMC article.
-
A lasting symbiosis: how the Hawaiian bobtail squid finds and keeps its bioluminescent bacterial partner.Nat Rev Microbiol. 2021 Oct;19(10):666-679. doi: 10.1038/s41579-021-00567-y. Epub 2021 Jun 4. Nat Rev Microbiol. 2021. PMID: 34089010 Free PMC article. Review.
-
A peptidoglycan-recognition protein orchestrates the first steps of symbiont recruitment in the squid-vibrio symbiosis.Symbiosis. 2022 May;87(1):31-43. doi: 10.1007/s13199-022-00855-y. Epub 2022 Aug 6. Symbiosis. 2022. PMID: 36177150 Free PMC article.
-
Independent host- and bacterium-based determinants protect a model symbiosis from phage predation.Cell Rep. 2022 Feb 15;38(7):110376. doi: 10.1016/j.celrep.2022.110376. Cell Rep. 2022. PMID: 35172163 Free PMC article.
-
Animal development in the microbial world: The power of experimental model systems.Curr Top Dev Biol. 2021;141:371-397. doi: 10.1016/bs.ctdb.2020.10.002. Epub 2020 Nov 24. Curr Top Dev Biol. 2021. PMID: 33602493 Free PMC article. Review.
References
-
- Altura MA, Heath-Heckman EA, Gillette A, Kremer N, Krachler AM, Brennan C, … McFall-Ngai MJ (2013). The first engagement of partners in the Euprymna scolopes-Vibrio fischeri symbiosis is a two-step process initiated by a few environmental symbiont cells. Environmental Microbiology, 15, 2937–2950. doi: 10.1111/1462-2920.12179. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

