Prevalence of Antibodies to Crimean-Congo Hemorrhagic Fever Virus in Ruminants, Nigeria, 2015
- PMID: 32186489
- PMCID: PMC7101109
- DOI: 10.3201/eid2604.190354
Prevalence of Antibodies to Crimean-Congo Hemorrhagic Fever Virus in Ruminants, Nigeria, 2015
Abstract
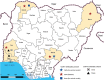
Crimean-Congo hemorrhagic fever virus (CCHFV) is a highly transmissible human pathogen. Infection is often misdiagnosed, in part because of poor availability of data in disease-endemic areas. We sampled 150 apparently healthy ruminants throughout Nigeria for virus seropositivity and detected virus-specific IgG in cattle (24%) and goats (2%), highlighting the need for further investigations.
Keywords: CCHF; CCHFV; Crimean-Congo hemorrhagic fever; Crimean-Congo hemorrhagic fever virus; Nigeria; antibodies; prevalence; ruminants; viruses; zoonoses.
Figures
References
-
- Mehrabi-Tavana A, Chinikar S, Mazaheri V. The seroepidemiological aspect of Crimean-Congo haemorrhagic fever in three health workers: a report from Iran. Arch Iran Med. 2002;15:255–8.
-
- Umoh JU, Ezeokoli CD, Ogwu D. Prevalence of antibodies to Crimean-haemorrhagic fever-Congo virus in cattle in northern Nigeria. Int J Zoonoses. 1983;10:151–4. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

