Muscle metabolomics analysis reveals potential biomarkers of exercise‑dependent improvement of the diaphragm function in chronic obstructive pulmonary disease
- PMID: 32186768
- PMCID: PMC7169662
- DOI: 10.3892/ijmm.2020.4537
Muscle metabolomics analysis reveals potential biomarkers of exercise‑dependent improvement of the diaphragm function in chronic obstructive pulmonary disease
Abstract
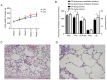
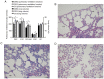
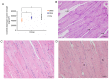
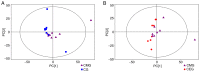
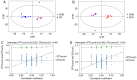
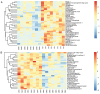
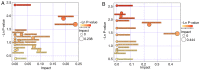
Decreased diaphragm function is a crucial factor leading to reduced ventilatory efficiency and worsening of quality of life in chronic obstructive pulmonary disease (COPD). Exercise training has been demonstrated to effectively improve the function of the diaphragm. However, the mechanism of this process has not been identified. The emergence of metabolomics has allowed the exploration of new ideas. The present study aimed to analyze the potential biomarkers of exercise‑dependent enhancement of diaphragm function in COPD using metabolomics. Sprague Dawley rats were divided into three groups: COPD + exercise group (CEG); COPD model group (CMG); and control group (CG). The first two groups were exposed to cigarette smoke for 16 weeks to establish a COPD model. Then, the rats in the CEG underwent aerobic exercise training for 9 weeks. Following confirmation that exercise effectively improved the diaphragm function, a gas chromatography tandem time‑of‑flight mass spectrometry analysis system was used to detect the differential metabolites and associated pathways in the diaphragm muscles of the different groups. Following exercise intervention, the pulmonary function and diaphragm contractility of the CEG rats were significantly improved compared with those of the CMG rats. A total of 36 different metabolites were identified in the comparison between the CMG and the CG. Pathway enrichment analysis indicated that these different metabolites were involved in 17 pathways. A total of 29 different metabolites were identified in the comparison between the CMG and the CEG, which are involved in 14 pathways. Candidate biomarkers were selected, and the pathways analysis of these metabolites demonstrated that 2 types of metabolic pathways, the nicotinic acid and nicotinamide metabolism and arginine and proline metabolism pathways, were associated with exercise‑induced pulmonary rehabilitation.
Figures

References
-
- Hodgev VA, Kostianev SS. Maximal inspiratory pressure predicts mortality in patients with chronic obstructive pulmonary disease in a five-year follow-up. Folia Med. 2006;48:36–41. - PubMed

