Blood Eosinophil Counts in Clinical Trials for Chronic Obstructive Pulmonary Disease
- PMID: 32186896
- PMCID: PMC7462391
- DOI: 10.1164/rccm.201912-2384PP
Blood Eosinophil Counts in Clinical Trials for Chronic Obstructive Pulmonary Disease
Figures
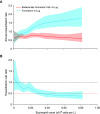
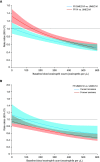
Comment in
-
Blood Eosinophil-directed Management of Airway Disease. The Past, Present, and Future.Am J Respir Crit Care Med. 2020 Sep 1;202(5):637-639. doi: 10.1164/rccm.202004-1013ED. Am J Respir Crit Care Med. 2020. PMID: 32356667 Free PMC article. No abstract available.
References
-
- Singh D, Martin M. Biologics for chronic obstructive pulmonary disease: present and future. BRN Reviews. 2018;4:34–52.
-
- FDA-NIH Biomarker Working Group. BEST (Biomarkers, EndpointS, and other Tools) resource. Silver Spring, MD: Food and Drug Administration (US); 2016. - PubMed
-
- European Medicines Agency. Amsterdam, the Netherlands: European Medicines Agency; 2014. Qualification of novel methodologies for medicine development: guidance to applicants.
-
- Food and Drug Administration. Silver Spring, MD: Food and Drug Administration (US); 2018. Biomarker qualification: evidentiary framework: guidance for industry and FDA staff.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

