Selumetinib in Children with Inoperable Plexiform Neurofibromas
- PMID: 32187457
- PMCID: PMC7305659
- DOI: 10.1056/NEJMoa1912735
Selumetinib in Children with Inoperable Plexiform Neurofibromas
Erratum in
-
Selumetinib in Children with Inoperable Plexiform Neurofibromas.N Engl J Med. 2020 Sep 24;383(13):1290. doi: 10.1056/NEJMx200013. N Engl J Med. 2020. PMID: 32966737 No abstract available.
Abstract
Background: No approved therapies exist for inoperable plexiform neurofibromas in patients with neurofibromatosis type 1.
Methods: We conducted an open-label, phase 2 trial of selumetinib to determine the objective response rate among patients with plexiform neurofibromas and to assess clinical benefit. Children with neurofibromatosis type 1 and symptomatic inoperable plexiform neurofibromas received oral selumetinib twice daily at a dose of 25 mg per square meter of body-surface area on a continuous dosing schedule (28-day cycles). Volumetric magnetic resonance imaging and clinical outcome assessments (pain, quality of life, disfigurement, and function) were performed at least every four cycles. Children rated tumor pain intensity on a scale from 0 (no pain) to 10 (worst pain imaginable).
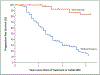
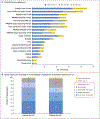
Results: A total of 50 children (median age, 10.2 years; range, 3.5 to 17.4) were enrolled from August 2015 through August 2016. The most frequent neurofibroma-related symptoms were disfigurement (44 patients), motor dysfunction (33), and pain (26). A total of 35 patients (70%) had a confirmed partial response as of March 29, 2019, and 28 of these patients had a durable response (lasting ≥1 year). After 1 year of treatment, the mean decrease in child-reported tumor pain-intensity scores was 2 points, considered a clinically meaningful improvement. In addition, clinically meaningful improvements were seen in child-reported and parent-reported interference of pain in daily functioning (38% and 50%, respectively) and overall health-related quality of life (48% and 58%, respectively) as well as in functional outcomes of strength (56% of patients) and range of motion (38% of patients). Five patients discontinued treatment because of toxic effects possibly related to selumetinib, and 6 patients had disease progression. The most frequent toxic effects were nausea, vomiting, or diarrhea; an asymptomatic increase in the creatine phosphokinase level; acneiform rash; and paronychia.
Conclusions: In this phase 2 trial, most children with neurofibromatosis type 1 and inoperable plexiform neurofibromas had durable tumor shrinkage and clinical benefit from selumetinib. (Funded by the Intramural Research Program of the National Institutes of Health and others; ClinicalTrials.gov number, NCT01362803.).
Copyright © 2020 Massachusetts Medical Society.
Figures

Comment in
-
New therapy for children with plexiform neurofibromas.Lancet Oncol. 2020 May;21(5):e238. doi: 10.1016/S1470-2045(20)30206-0. Epub 2020 Mar 26. Lancet Oncol. 2020. PMID: 32222155 No abstract available.
-
Selumetinib benefits children with inoperable plexiform neurofibromas.Nat Rev Clin Oncol. 2020 May;17(5):273. doi: 10.1038/s41571-020-0361-7. Nat Rev Clin Oncol. 2020. PMID: 32235897 No abstract available.
References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
