Age attenuates the T-type CaV 3.2-RyR axis in vascular smooth muscle
- PMID: 32187825
- PMCID: PMC7189999
- DOI: 10.1111/acel.13134
Age attenuates the T-type CaV 3.2-RyR axis in vascular smooth muscle
Abstract
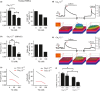
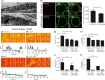
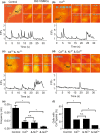
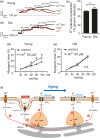
Caveolae position CaV 3.2 (T-type Ca2+ channel encoded by the α-3.2 subunit) sufficiently close to RyR (ryanodine receptors) for extracellular Ca2+ influx to trigger Ca2+ sparks and large-conductance Ca2+ -activated K+ channel feedback in vascular smooth muscle. We hypothesize that this mechanism of Ca2+ spark generation is affected by age. Using smooth muscle cells (VSMCs) from mouse mesenteric arteries, we found that both Cav 3.2 channel inhibition by Ni2+ (50 µM) and caveolae disruption by methyl-ß-cyclodextrin or genetic abolition of Eps15 homology domain-containing protein (EHD2) inhibited Ca2+ sparks in cells from young (4 months) but not old (12 months) mice. In accordance, expression of Cav 3.2 channel was higher in mesenteric arteries from young than old mice. Similar effects were observed for caveolae density. Using SMAKO Cav 1.2-/- mice, caffeine (RyR activator) and thapsigargin (Ca2+ transport ATPase inhibitor), we found that sufficient SR Ca2+ load is a prerequisite for the CaV 3.2-RyR axis to generate Ca2+ sparks. We identified a fraction of Ca2+ sparks in aged VSMCs, which is sensitive to the TRP channel blocker Gd3+ (100 µM), but insensitive to CaV 1.2 and CaV 3.2 channel blockade. Our data demonstrate that the VSMC CaV 3.2-RyR axis is down-regulated by aging. This defective CaV 3.2-RyR coupling is counterbalanced by a Gd3+ sensitive Ca2+ pathway providing compensatory Ca2+ influx for triggering Ca2+ sparks in aged VSMCs.
Keywords: T-type calcium channels; aging; calcium sparks; caveolae; ryanodine receptors; vascular smooth muscle.
© 2020 The Authors. Aging Cell published by the Anatomical Society and John Wiley & Sons Ltd.
Conflict of interest statement
None declared.
Figures


Similar articles
-
Senolytics prevent caveolar CaV 3.2-RyR axis malfunction in old vascular smooth muscle.Aging Cell. 2023 Nov;22(11):e14002. doi: 10.1111/acel.14002. Epub 2023 Oct 14. Aging Cell. 2023. PMID: 37837625 Free PMC article.
-
Differential targeting and signalling of voltage-gated T-type Cav 3.2 and L-type Cav 1.2 channels to ryanodine receptors in mesenteric arteries.J Physiol. 2018 Oct;596(20):4863-4877. doi: 10.1113/JP276923. Epub 2018 Sep 15. J Physiol. 2018. PMID: 30146760 Free PMC article.
-
Caveolae Link CaV3.2 Channels to BKCa-Mediated Feedback in Vascular Smooth Muscle.Arterioscler Thromb Vasc Biol. 2018 Oct;38(10):2371-2381. doi: 10.1161/ATVBAHA.118.311394. Arterioscler Thromb Vasc Biol. 2018. PMID: 30354206
-
Elementary calcium signaling in arterial smooth muscle.Channels (Austin). 2019 Dec;13(1):505-519. doi: 10.1080/19336950.2019.1688910. Channels (Austin). 2019. PMID: 31797713 Free PMC article. Review.
-
Role of ryanodine receptor subtypes in initiation and formation of calcium sparks in arterial smooth muscle: comparison with striated muscle.J Biomed Biotechnol. 2009;2009:135249. doi: 10.1155/2009/135249. Epub 2009 Dec 8. J Biomed Biotechnol. 2009. PMID: 20029633 Free PMC article. Review.
Cited by
-
Myogenic Vasoconstriction Requires Canonical Gq/11 Signaling of the Angiotensin II Type 1 Receptor.J Am Heart Assoc. 2022 Feb 15;11(4):e022070. doi: 10.1161/JAHA.121.022070. Epub 2022 Feb 8. J Am Heart Assoc. 2022. PMID: 35132870 Free PMC article.
-
Senolytics prevent caveolar CaV 3.2-RyR axis malfunction in old vascular smooth muscle.Aging Cell. 2023 Nov;22(11):e14002. doi: 10.1111/acel.14002. Epub 2023 Oct 14. Aging Cell. 2023. PMID: 37837625 Free PMC article.
-
Vascular calcium signalling and ageing.J Physiol. 2021 Dec;599(24):5361-5377. doi: 10.1113/JP280950. Epub 2021 Nov 21. J Physiol. 2021. PMID: 34705288 Free PMC article. Review.
-
Aging-Induced Impairment of Vascular Function: Mitochondrial Redox Contributions and Physiological/Clinical Implications.Antioxid Redox Signal. 2021 Oct 20;35(12):974-1015. doi: 10.1089/ars.2021.0031. Epub 2021 Sep 17. Antioxid Redox Signal. 2021. PMID: 34314229 Free PMC article. Review.
-
Impact of aging on vascular ion channels: perspectives and knowledge gaps across major organ systems.Am J Physiol Heart Circ Physiol. 2023 Nov 1;325(5):H1012-H1038. doi: 10.1152/ajpheart.00288.2023. Epub 2023 Aug 25. Am J Physiol Heart Circ Physiol. 2023. PMID: 37624095 Free PMC article. Review.
References
-
- Abd El‐Rahman, R. R. , Harraz, O. F. , Brett, S. E. , Anfinogenova, Y. , Mufti, R. E. , Goldman, D. , & Welsh, D. G. (2013). Identification of L‐ and T‐type Ca2+ channels in rat cerebral arteries: Role in myogenic tone development. American Journal of Physiology Heart and Circulatory Physiology, 304, H58–H71. - PMC - PubMed
-
- Bakircioglu, M. E. , Sievert, K.‐D. , Nunes, L. , Lau, A. , Lin, C.‐S. , & Lue, T. F. (2001). Decreased trabecular smooth muscle and caveolin‐1 expression in the penile tissue of aged rats. The Journal of Urology, 166, 734–738. - PubMed
-
- Bergdahl, A. , & Sward, K. (2004). Caveolae‐associated signalling in smooth muscle. Canadian Journal of Physiology and Pharmacology, 82, 289–299. - PubMed
-
- Berrier, C. , Coulombe, A. , Szabo, I. , Zoratti, M. , & Ghazi, A. (1992). Gadolinium ion inhibits loss of metabolites induced by osmotic shock and large stretch‐activated channels in bacteria. European Journal of Biochemistry, 206, 559–565. - PubMed
-
- Boersma, E. , Poldermans, D. , Bax, J. J. , Steyerberg, E. W. , Thomson, I. R. , Banga, J. D. , … Group DS (2001). Predictors of cardiac events after major vascular surgery: Role of clinical characteristics, dobutamine echocardiography, and β‐blocker therapy. JAMA, 285, 1865–1873. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

