Impact of repeated cycles of EGF bispecific angiotoxin (eBAT) administered at a reduced interval from doxorubicin chemotherapy in dogs with splenic haemangiosarcoma
- PMID: 32187827
- PMCID: PMC7501152
- DOI: 10.1111/vco.12590
Impact of repeated cycles of EGF bispecific angiotoxin (eBAT) administered at a reduced interval from doxorubicin chemotherapy in dogs with splenic haemangiosarcoma
Abstract
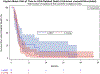
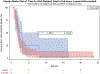
We previously reported that eBAT, an EGF-targeted angiotoxin, was safe and it improved the overall survival for dogs with splenic haemangiosarcoma when added to the standard of care in a single cycle of three administrations in the minimal residual disease setting. Our objective for the SRCBST-2 trial was to assess whether increased dosing through multiple cycles of eBAT would be well tolerated and would further enhance the benefits of eBAT. Eligibility was expanded to dogs with stage 3 haemangiosarcoma, provided that gross lesions could be surgically excised. The interval between eBAT and the start of chemotherapy was reduced, and the experimental therapy was expanded to three cycles, each administered at the biologically active dose (50 μg/kg) on a Monday/Wednesday/Friday schedule following splenectomy, and scheduled 1 week prior to the first, second and fifth doxorubicin chemotherapy. Twenty-five dogs were enrolled; six experienced acute hypotension with two requiring hospitalization. Self-limiting elevation of ALT was observed in one dog. A statistically significant survival benefit was not seen in this study in eBAT-treated dogs compared with a Contemporary comparison group of dogs with stages 1-3 haemangiosarcoma treated with standard of care alone. Our results indicate that repeated dosing cycles of eBAT starting 1 week prior to doxorubicin chemotherapy led to greater toxicity and reduced efficacy compared with a single cycle given between surgery and a delayed start of chemotherapy. Further work is needed to understand the precise mechanisms of action of eBAT in order to optimize its clinical benefits in the treatment of canine haemangiosarcoma and other tumours. IACUC Protocols 1110A06186 and 1507-32804A.
Keywords: canine; epidermal growth factor receptor; haemangiosarcoma; sarcoma; targeted toxin; urokinase plasminogen activator receptor.
© 2020 John Wiley & Sons Ltd.
Conflict of interest statement
Conflict of Interest
The authors declare that patent “Reduction of EGFR therapeutic toxicity,” related to this work and listing JFM, DV, and AB as inventors, has been filed by the University of Minnesota Office of Technology Commercialization. Anivive Lifesciences owns the license for eBAT for all non-human species and applications.
Figures



Similar articles
-
Bispecific Targeting of EGFR and Urokinase Receptor (uPAR) Using Ligand-Targeted Toxins in Solid Tumors.Biomolecules. 2020 Jun 25;10(6):956. doi: 10.3390/biom10060956. Biomolecules. 2020. PMID: 32630411 Free PMC article. Review.
-
Safe and Effective Sarcoma Therapy through Bispecific Targeting of EGFR and uPAR.Mol Cancer Ther. 2017 May;16(5):956-965. doi: 10.1158/1535-7163.MCT-16-0637. Epub 2017 Feb 13. Mol Cancer Ther. 2017. PMID: 28193671 Free PMC article.
-
Does thalidomide prolong survival in dogs with splenic haemangiosarcoma?J Small Anim Pract. 2018 Feb;59(2):85-91. doi: 10.1111/jsap.12796. Epub 2017 Dec 6. J Small Anim Pract. 2018. PMID: 29210452
-
Doxorubicin and deracoxib adjuvant therapy for canine splenic hemangiosarcoma: a pilot study.Can Vet J. 2013 Mar;54(3):237-42. Can Vet J. 2013. PMID: 23997259 Free PMC article. Clinical Trial.
-
Treatment of canine hemangiosarcoma: 2000 and beyond.J Vet Intern Med. 2000 Sep-Oct;14(5):479-85. doi: 10.1892/0891-6640(2000)014<0479:tochab>2.3.co;2. J Vet Intern Med. 2000. PMID: 11012108 Review.
Cited by
-
Bispecific Targeting of EGFR and Urokinase Receptor (uPAR) Using Ligand-Targeted Toxins in Solid Tumors.Biomolecules. 2020 Jun 25;10(6):956. doi: 10.3390/biom10060956. Biomolecules. 2020. PMID: 32630411 Free PMC article. Review.
-
Targeting Receptors on Cancer Cells with Protein Toxins.Biomolecules. 2020 Sep 17;10(9):1331. doi: 10.3390/biom10091331. Biomolecules. 2020. PMID: 32957689 Free PMC article. Review.
-
Toxicity Profile of eBAT, a Bispecific Ligand-Targeted Toxin Directed to EGFR and uPAR, in Mice and a Clinical Dog Model.Toxins (Basel). 2024 Aug 26;16(9):376. doi: 10.3390/toxins16090376. Toxins (Basel). 2024. PMID: 39330834 Free PMC article.
-
Diagnosis, Prognosis, and Treatment of Canine Hemangiosarcoma: A Review Based on a Consensus Organized by the Brazilian Association of Veterinary Oncology, ABROVET.Cancers (Basel). 2023 Mar 29;15(7):2025. doi: 10.3390/cancers15072025. Cancers (Basel). 2023. PMID: 37046686 Free PMC article. Review.
-
Younger Age Is Associated with Favorable Outcomes in Adult Dogs with Hemangiosarcoma Receiving Adjuvant Doxorubicin Chemotherapy: Results from the PRO-DOX Study.Res Sq [Preprint]. 2025 May 5:rs.3.rs-6573099. doi: 10.21203/rs.3.rs-6573099/v1. Res Sq. 2025. PMID: 40386412 Free PMC article. Preprint.
References
Publication types
MeSH terms
Substances
Grants and funding
- CETI Translational Award/Masonic Cancer Center, UMN
- Hyundai Scholar Senior Research Award/Hyundai Hope On Wheels
- P30 CA077598/CA/NCI NIH HHS/United States
- Angiosarcoma Awareness Foundation
- R01 CA036725/CA/NCI NIH HHS/United States
- R01 CA36725/NCI NIAID
- K01OD017242/National Institute of Health
- GREYlong
- Animal Cancer Care and Research Program, UMN
- Masonic Cancer Center, University of Minnesota Sarcoma Translational Working Group
- 1889-G/American Kennel Club Canine Health Foundation
- K01 OD017242/OD/NIH HHS/United States
- Randy Shaver Cancer Research and Community Foundation
- UL1 TR002494/TR/NCATS NIH HHS/United States
- AB15MN-002 f/National Canine Cancer Foundation
LinkOut - more resources
Full Text Sources
Research Materials

