Light-Driven ATP Regeneration in Diblock/Grafted Hybrid Vesicles
- PMID: 32187828
- PMCID: PMC7496644
- DOI: 10.1002/cbic.201900774
Light-Driven ATP Regeneration in Diblock/Grafted Hybrid Vesicles
Abstract
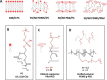
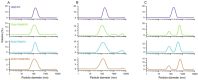
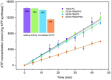
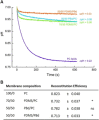
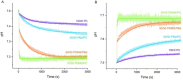
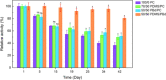
Light-driven ATP regeneration systems combining ATP synthase and bacteriorhodopsin have been proposed as an energy supply in the field of synthetic biology. Energy is required to power biochemical reactions within artificially created reaction compartments like protocells, which are typically based on either lipid or polymer membranes. The insertion of membrane proteins into different hybrid membranes is delicate, and studies comparing these systems with liposomes are needed. Here we present a detailed study of membrane protein functionality in different hybrid compartments made of graft polymer PDMS-g-PEO and diblock copolymer PBd-PEO. Activity of more than 90 % in lipid/polymer-based hybrid vesicles could prove an excellent biocompatibility. A significant enhancement of long-term stability (80 % remaining activity after 42 days) could be demonstrated in polymer/polymer-based hybrids.
Keywords: ATP synthase; bacteriorhodopsin; diblock polymers; energy conversion; graft polymers; permeability.
© 2020 The Authors. Published by Wiley-VCH Verlag GmbH & Co. KGaA.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Toward Artificial Mitochondrion: Mimicking Oxidative Phosphorylation in Polymer and Hybrid Membranes.Nano Lett. 2017 Nov 8;17(11):6816-6821. doi: 10.1021/acs.nanolett.7b03093. Epub 2017 Oct 27. Nano Lett. 2017. PMID: 29067800
-
Hybrid copolymer-phospholipid vesicles: phase separation resembling mixed phospholipid lamellae, but with mechanical stability and control.Soft Matter. 2015 Apr 7;11(13):2617-26. doi: 10.1039/c4sm02502d. Soft Matter. 2015. PMID: 25687473
-
Increased efficiency of charge-mediated fusion in polymer/lipid hybrid membranes.Proc Natl Acad Sci U S A. 2022 May 17;119(20):e2122468119. doi: 10.1073/pnas.2122468119. Epub 2022 May 12. Proc Natl Acad Sci U S A. 2022. PMID: 35549547 Free PMC article.
-
Lipid-polymer hybrid nanoparticles as a new generation therapeutic delivery platform: a review.Eur J Pharm Biopharm. 2013 Nov;85(3 Pt A):427-43. doi: 10.1016/j.ejpb.2013.07.002. Epub 2013 Jul 17. Eur J Pharm Biopharm. 2013. PMID: 23872180 Review.
-
Biopores/membrane proteins in synthetic polymer membranes.Biochim Biophys Acta Biomembr. 2017 Apr;1859(4):619-638. doi: 10.1016/j.bbamem.2016.10.015. Epub 2016 Oct 29. Biochim Biophys Acta Biomembr. 2017. PMID: 27984019 Review.
Cited by
-
Advancing synthetic biology through cell-free protein synthesis.Comput Struct Biotechnol J. 2023 May 4;21:2899-2908. doi: 10.1016/j.csbj.2023.05.003. eCollection 2023. Comput Struct Biotechnol J. 2023. PMID: 37216017 Free PMC article. Review.
-
Current Perspectives on Synthetic Compartments for Biomedical Applications.Int J Mol Sci. 2022 May 20;23(10):5718. doi: 10.3390/ijms23105718. Int J Mol Sci. 2022. PMID: 35628527 Free PMC article. Review.
-
Advances in block copolymer-phospholipid hybrid vesicles: from physical-chemical properties to applications.Chem Sci. 2024 Jun 24;15(28):10724-10744. doi: 10.1039/d4sc01444h. eCollection 2024 Jul 17. Chem Sci. 2024. PMID: 39027291 Free PMC article. Review.
-
Hybrid polymer/lipid vesicles: Influence of polymer architecture and molar mass on line tension.Biophys J. 2022 Jan 4;121(1):61-67. doi: 10.1016/j.bpj.2021.12.005. Epub 2021 Dec 8. Biophys J. 2022. PMID: 34890579 Free PMC article.
-
Spatiotemporal control of signal-driven enzymatic reaction in artificial cell-like polymersomes.Nat Commun. 2022 Sep 2;13(1):5179. doi: 10.1038/s41467-022-32889-7. Nat Commun. 2022. PMID: 36056018 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

