Bone augmentation by octacalcium phosphate and collagen composite coated with poly-lactic acid cage
- PMID: 32187863
- PMCID: PMC7453772
- DOI: 10.1002/cre2.287
Bone augmentation by octacalcium phosphate and collagen composite coated with poly-lactic acid cage
Abstract
Objective: Although octacalcium phosphate and collagen composite (OCP/Col) has demonstrated excellent bone regeneration, it has never achieved bone augmentation. The present study investigated whether it could be enabled by OCP/Col disks treated with parathyroid hormone (PTH) and covered with a poly-lactic acid (PLA) cage.
Materials and methods: The prepared OCP/Col disks with three different types of PLA cages (no hole, one large hole, several small holes) were implanted into subperiosteal pockets in rodent calvaria. Histological, and histomorphometric analyses were conducted at 12 weeks after implantation.
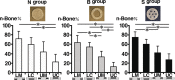
Results: Implants with all PLA cage variants achieved sufficient bone augmentation, and analyses showed that new bone was formed from the original bone and along the PLA cage. While the PLA cage variant with no holes sporadically evoked new bone formation even at the central area of the roof of the PLA cage, the PLA cage variants with holes had no new bone in the area of the hole or beneath the periosteum.
Conclusions: These results suggest that sufficient bone augmentation could be achieved by treating the OCP/Col disks with PTH and covering them with a PLA cage, and periosteum might not have been involved in the bone formation in this experiment.
Keywords: bone regeneration; collagen; octacalcium phosphate; parathyroid hormone; poly-lactic acid.
© 2020 The Authors. Clinical and Experimental Dental Research published by John Wiley & Sons Ltd.
Conflict of interest statement
One of the authors (S.K.) has obtained patents on OCP/Col in Japan (#5046511) and combination of calcium phosphate containing porous composite and PTH in Japan (#6094716).
Figures







References
-
- Brown, W. E. , Mathew, M. , & Tung, M. S. (1981). Crystal‐chemistry of octacalcium phosphate. Progress in Crystal Growth and Characterization of Materials, 4(1–2), 59–87. 10.1016/0146-3535(81)90048-4 - DOI
-
- Brown, W. E. , Smith, J. P. , Frazier, A. W. , & Lehr, J. R. (1962). Crystallographic and chemical relations between octacalcium phosphate and hydroxyapatite. Nature, 196(4859), 1050–1055. 10.1038/1961050a0 - DOI
-
- Buser, D. , Martin, W. , & Belser, U. C. (2004). Optimizing esthetics for implant restorations in the anterior maxilla: Anatomic and surgical considerations. The International Journal of Oral & Maxillofacial Implants, 19(Suppl), 43–61. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

