Correlative Monitoring of Immune Activation and Tissue Damage in Malignant Melanoma-An Algorithm for Identification of Tolerance Breakage During Immune Checkpoint Inhibitor Therapy of Cancer
- PMID: 32188047
- PMCID: PMC7139313
- DOI: 10.3390/ijms21062020
Correlative Monitoring of Immune Activation and Tissue Damage in Malignant Melanoma-An Algorithm for Identification of Tolerance Breakage During Immune Checkpoint Inhibitor Therapy of Cancer
Abstract
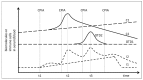
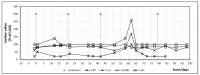
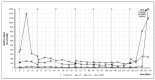
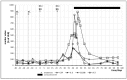
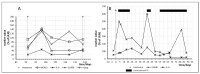
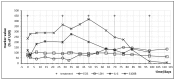
We describe an innovative approach for identification of tolerance breakage during immune checkpoint inhibitor therapy in malignant melanoma. Checkpoint inhibitor therapy enhances the immunologic clearance of cancer by suppressing pathways which induce immune suppression and tolerance. We posit that by analyzing temporal correlations of key markers of immune activation and tissue damage it would be possible to detect the onset of anticancer immune reaction as well as of immunologic adverse effects which might become crucial for optimization as well as safety of immune checkpoint inhibitor treatment. We analyzed time courses of routine laboratory values of serum tumor markers as well as of markers of immune activation in 17 patients with metastasized malignant melanoma receiving checkpoint inhibition and weekly laboratory controls. A parallel serum level increase of interleukin-6 and the tumor marker S100B could be identified in 13 patients, suggesting that the onset of tolerance breakage under checkpoint inhibition may be identified and measured. Immune-related adverse events in the patients were also accompanied by a peak of IL-6. In six patients, the onset of a putative anticancer immune reaction and the beginning of immunologic adverse events occurred in the same treatment cycle; in six patients the immunologic adverse reactions took place in separate cycles.
Keywords: S100B; checkpoint inhibitor therapy; eosinophils; interleukin 6; irAE; macrophages; malignant melanoma; temporal correlation; tolerance breakage; treatment monitoring.
Conflict of interest statement
Albert Rübben has received travel grants and lecture fees from Bristol-Myers Squibb, MSD, Amgen and Roche Pharma. Renate U. Wahl, Arturo Araujo and Marike Leijs declare no conflict of interest.
Figures


Similar articles
-
Immune signature as predictive marker for response to checkpoint inhibitor immunotherapy and overall survival in melanoma.Cancer Med. 2021 Mar;10(5):1562-1575. doi: 10.1002/cam4.3710. Epub 2021 Jan 15. Cancer Med. 2021. PMID: 33449393 Free PMC article.
-
Use of immunotherapy and surgery for stage IV melanoma.Cancer. 2020 Jun 1;126(11):2614-2624. doi: 10.1002/cncr.32817. Epub 2020 Mar 10. Cancer. 2020. PMID: 32157676
-
Immunotherapy in the Treatment of Metastatic Melanoma: Current Knowledge and Future Directions.J Immunol Res. 2020 Jun 28;2020:9235638. doi: 10.1155/2020/9235638. eCollection 2020. J Immunol Res. 2020. PMID: 32671117 Free PMC article. Review.
-
Role of immune checkpoint inhibitors in the revolutionization of advanced melanoma care.Int Immunopharmacol. 2020 Jun;83:106417. doi: 10.1016/j.intimp.2020.106417. Epub 2020 Mar 19. Int Immunopharmacol. 2020. PMID: 32200155 Review.
-
Immune checkpoint inhibitors in ocular melanomas: contrasting efficacy with cutaneous melanomas.Immunotherapy. 2020 Nov;12(16):1149-1152. doi: 10.2217/imt-2020-0234. Epub 2020 Oct 20. Immunotherapy. 2020. PMID: 33076742 No abstract available.
Cited by
-
Proteomics to study cancer immunity and improve treatment.Semin Immunopathol. 2023 Mar;45(2):241-251. doi: 10.1007/s00281-022-00980-2. Epub 2023 Jan 4. Semin Immunopathol. 2023. PMID: 36598558 Free PMC article. Review.
-
Biomarkers associated with immune-related adverse events induced by immune checkpoint inhibitors.World J Clin Oncol. 2024 Aug 24;15(8):1002-1020. doi: 10.5306/wjco.v15.i8.1002. World J Clin Oncol. 2024. PMID: 39193157 Free PMC article. Review.
-
Hypothalamic gliosis as a potential mediator of improved glucose tolerance induced by time-restricted feeding in obese mice.Am J Physiol Cell Physiol. 2025 Sep 1;329(3):C834-C847. doi: 10.1152/ajpcell.00357.2025. Epub 2025 Jul 31. Am J Physiol Cell Physiol. 2025. PMID: 40741982 Free PMC article.
-
Converged Rab37/IL-6 trafficking and STAT3/PD-1 transcription axes elicit an immunosuppressive lung tumor microenvironment.Theranostics. 2021 May 12;11(14):7029-7044. doi: 10.7150/thno.60040. eCollection 2021. Theranostics. 2021. PMID: 34093869 Free PMC article.
-
Skin Infiltrate Composition as a Telling Measure of Responses to Checkpoint Inhibitors.JID Innov. 2023 Feb 9;3(5):100190. doi: 10.1016/j.xjidi.2023.100190. eCollection 2023 Sep. JID Innov. 2023. PMID: 37554516 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

