The Impact of Natural Compounds on S-Shaped Aβ42 Fibril: From Molecular Docking to Biophysical Characterization
- PMID: 32188076
- PMCID: PMC7139307
- DOI: 10.3390/ijms21062017
The Impact of Natural Compounds on S-Shaped Aβ42 Fibril: From Molecular Docking to Biophysical Characterization
Abstract
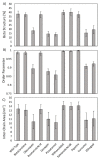
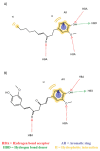
The pursuit for effective strategies inhibiting the amyloidogenic process in neurodegenerative disorders, such as Alzheimer's disease (AD), remains one of the main unsolved issues, and only a few drugs have demonstrated to delay the degeneration of the cognitive system. Moreover, most therapies induce severe side effects and are not effective at all stages of the illness. The need to find novel and reliable drugs appears therefore of primary importance. In this context, natural compounds have shown interesting beneficial effects on the onset and progression of neurodegenerative diseases, exhibiting a great inhibitory activity on the formation of amyloid aggregates and proving to be effective in many preclinical and clinical studies. However, their inhibitory mechanism is still unclear. In this work, ensemble docking and molecular dynamics simulations on S-shaped Aβ42 fibrils have been carried out to evaluate the influence of several natural compounds on amyloid conformational behaviour. A deep understanding of the interaction mechanisms between natural compounds and Aβ aggregates may play a key role to pave the way for design, discovery and optimization strategies toward an efficient destabilization of toxic amyloid assemblies.
Keywords: Alzheimer’s disease; Amyloid β; S-shape; ensemble docking; molecular dynamics; natural compounds.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Cohen S.I.A., Linse S., Luheshi L.M., Hellstrand E., White D.A., Rajah L., Otzen D.E., Vendruscolo M., Dobson C.M., Knowles T.P.J. Proliferation of amyloid- 42 aggregates occurs through a secondary nucleation mechanism. Proc. Natl. Acad. Sci. USA. 2013;110:9758–9763. doi: 10.1073/pnas.1218402110. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

