Role of Autophagy in Von Willebrand Factor Secretion by Endothelial Cells and in the In Vivo Thrombin-Antithrombin Complex Formation Promoted by the HIV-1 Matrix Protein p17
- PMID: 32188077
- PMCID: PMC7139864
- DOI: 10.3390/ijms21062022
Role of Autophagy in Von Willebrand Factor Secretion by Endothelial Cells and in the In Vivo Thrombin-Antithrombin Complex Formation Promoted by the HIV-1 Matrix Protein p17
Abstract
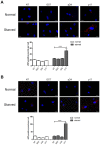
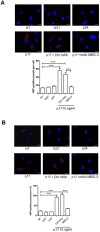
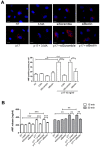
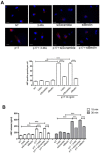
Although the advent of combined antiretroviral therapy has substantially improved the survival of HIV-1-infected individuals, non-AIDS-related diseases are becoming increasingly prevalent in HIV-1-infected patients. Persistent abnormalities in coagulation appear to contribute to excess risk for a broad spectrum of non-AIDS defining complications. Alterations in coagulation biology in the context of HIV infection seem to be largely a consequence of a chronically inflammatory microenvironment leading to endothelial cell (EC) dysfunction. A possible direct role of HIV-1 proteins in sustaining EC dysfunction has been postulated but not yet investigated. The HIV-1 matrix protein p17 (p17) is secreted from HIV-1-infected cells and is known to sustain inflammatory processes by activating ECs. The aim of this study was to investigate the possibility that p17-driven stimulation of human ECs is associated with increased production of critical coagulation factors. Here we show the involvement of autophagy in the p17-induced accumulation and secretion of von Willebrand factor (vWF) by ECs. In vivo experiments confirmed the capability of p17 to exert a potent pro-coagulant activity soon after its intravenous administration.
Keywords: AIDS-related diseases; HIV-1; autophagy; coagulation; p17 matrix protein; thrombin-antithrombin complex.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

