Pituitary Hyperplasia, Hormonal Changes and Prolactinoma Development in Males Exposed to Estrogens-An Insight From Translational Studies
- PMID: 32188093
- PMCID: PMC7139613
- DOI: 10.3390/ijms21062024
Pituitary Hyperplasia, Hormonal Changes and Prolactinoma Development in Males Exposed to Estrogens-An Insight From Translational Studies
Abstract
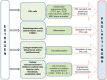
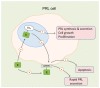
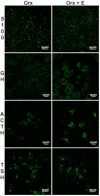
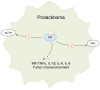
Estrogen signaling plays an important role in pituitary development and function. In sensitive rat or mice strains of both sexes, estrogen treatments promote lactotropic cell proliferation and induce the formation of pituitary adenomas (dominantly prolactin or growth-hormone-secreting ones). In male patients receiving estrogen, treatment does not necessarily result in pituitary hyperplasia, hyperprolactinemia or adenoma development. In this review, we comprehensively analyze the mechanisms of estrogen action upon their application in male animal models comparing it with available data in human subjects. Sex-specific molecular targets of estrogen action in lactotropic (PRL) cells are highlighted in the context of their proliferative and secretory activity. In addition, putative effects of estradiol on the cellular/tumor microenvironment and the contribution of postnatal pituitary progenitor/stem cells and transdifferentiation processes to prolactinoma development have been analyzed. Finally, estrogen-induced morphological and hormone-secreting changes in pituitary thyrotropic (TSH) and adrenocorticotropic (ACTH) cells are discussed, as well as the putative role of the thyroid and/or glucocorticoid hormones in prolactinoma development, based on the current scarce literature.
Keywords: adrenocorticotropic hormone; estrogen; folliculo-stellate cells; men; microenvironment; pituitary gland; prolactin; prolactinoma; rat; thyroid-stimulating hormone.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Three-dimensional imaging of hormone-secreting cells and their microvessel environment in estrogen-induced prolactinoma of the rat pituitary gland by confocal laser scanning microscopy.Appl Immunohistochem Mol Morphol. 2001 Dec;9(4):364-70. doi: 10.1097/00129039-200112000-00013. Appl Immunohistochem Mol Morphol. 2001. PMID: 11759065
-
Laminin inhibits lactotroph proliferation and is reduced in early prolactinoma development.Mol Cell Endocrinol. 2003 Sep 30;207(1-2):13-20. doi: 10.1016/s0303-7207(03)00237-5. Mol Cell Endocrinol. 2003. PMID: 12972179
-
Pit-1 gene expression in human pituitary adenomas using the reverse transcription polymerase chain reaction method.Clin Endocrinol (Oxf). 1996 Sep;45(3):263-72. doi: 10.1046/j.1365-2265.1996.00812.x. Clin Endocrinol (Oxf). 1996. PMID: 8949563
-
Pituitary prolactin-secreting tumor formation: recent developments.Biol Signals Recept. 2000 Jan-Feb;9(1):1-20. doi: 10.1159/000014618. Biol Signals Recept. 2000. PMID: 10686432 Review.
-
Relation among Aromatase P450 and Tumoral Growth in Human Prolactinomas.Int J Mol Sci. 2017 Nov 1;18(11):2299. doi: 10.3390/ijms18112299. Int J Mol Sci. 2017. PMID: 29104246 Free PMC article. Review.
Cited by
-
The Value of ER∝ in the Prognosis of GH- and PRL-Secreting PitNETs: Clinicopathological Correlations.Int J Mol Sci. 2023 Nov 10;24(22):16162. doi: 10.3390/ijms242216162. Int J Mol Sci. 2023. PMID: 38003353 Free PMC article.
-
Double PitNETs: A Case Report and Literature Review.Cancers (Basel). 2025 Feb 17;17(4):675. doi: 10.3390/cancers17040675. Cancers (Basel). 2025. PMID: 40002269 Free PMC article. Review.
-
Anastrozole as add-on therapy for cabergoline-resistant prolactin-secreting pituitary adenomas: real-life experience in male patients.Pituitary. 2021 Dec;24(6):914-921. doi: 10.1007/s11102-021-01165-0. Epub 2021 Jun 26. Pituitary. 2021. PMID: 34173929 Free PMC article.
-
Role of Estrogen and Estrogen Receptor in GH-Secreting Adenomas.Int J Mol Sci. 2023 Jun 8;24(12):9920. doi: 10.3390/ijms24129920. Int J Mol Sci. 2023. PMID: 37373068 Free PMC article. Review.
-
Molecular Pathways in Prolactinomas: Translational and Therapeutic Implications.Int J Mol Sci. 2021 Oct 18;22(20):11247. doi: 10.3390/ijms222011247. Int J Mol Sci. 2021. PMID: 34681905 Free PMC article. Review.
References
-
- Mitsui T., Ishida M., Izawa M., Arita J. Differences between rat strains in the development of PRL-secreting pituitary tumors with long-term estrogen treatment: In vitro insulin-like growth factor-1-induced lactotroph proliferation and gene expression are affected in Wistar-Kyoto rats with low estrogen-susceptibility. Endocr. J. 2013;60:1251–1259. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

