Unmet needs of chronic hepatitis C in the era of direct-acting antiviral therapy
- PMID: 32188235
- PMCID: PMC7364348
- DOI: 10.3350/cmh.2020.0018
Unmet needs of chronic hepatitis C in the era of direct-acting antiviral therapy
Abstract
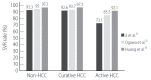
The treatment of chronic hepatitis C (CHC) has been revolutionized in an era of all-oral direct-acting antivirals (DAAs) since 2014. Satisfactory treatment efficacy and tolerability can be provided by novel DAAs. Nevertheless, there are still some unmet needs and emerging issues in the treatment of CHC in the DAA era. Certain hard-to-cure populations are prone to have inferior treatment responses, including patients with severe liver decompensation, active hepatocellular carcinoma (HCC), and hepatitis C virus (HCV) genotype 3 (HCV-3) infection and those who experience multiple DAA treatment failures. Hepatitis B virus (HBV) reactivation during and after DAA treatment has raised concern regarding the use of prophylactic antivirals against HBV throughout DAA treatment. However, the standard strategy for the use of prophylactic antivirals is not uniform across regional guidelines. In the post-sustained virological response (SVR) period, HCC still occurs in a substantial proportion of patients. Due to the relatively short follow-up period, the net benefit of the achievement of an SVR by DAAs in the reduction of extrahepatic manifestations has not yet been determined. Attention must also be paid to HCV reinfection, particularly in high-risk populations. The most critical and unmet need for HCV elimination is the large gap in the HCV care cascade at the population level. To accomplish the World Health Organization (WHO)'s goal for HCV elimination by 2030, the expansion of access to HCV care requires a continuous effort to overcome practical and political challenges.
Keywords: Direct-acting antivirals; Hepatitis C virus; Treatment.
Conflict of interest statement
Ming-Lung Yu: Research support (grant) from AbbVie, Abbott, BMS, Gilead, and Merck. Consultant for AbbVie, Abbott, Ascletis, BMS, Gilead, Merck and PharmaEssentia. Speaker for AbbVie, Abbott, Ascletis, BMS, Gilead, and Merck.
Chung-Feng Huang: Speaker for AbbVie, Abbott, BMS, Gilead, and Merck.
Figures
References
-
- Huang JF, Huang CF, Yeh ML, Dai CY, Yu ML, Chuang WL. Updates in the management and treatment of HCV genotype 3, what are the remaining challenges? Expert Rev Anti Infect Ther. 2018;16:907–912. - PubMed
-
- Foster GR, Afdhal N, Roberts SK, Bräu N, Gane EJ, Pianko S, et al. Sofosbuvir and velpatasvir for HCV genotype 2 and 3 infection. N Engl J Med. 2015;373:2608–2617. - PubMed
-
- Hezode C, Reau N, Svarovskaia ES, Doehle BP, Shanmugam R, Dvory-Sobol H, et al. Resistance analysis in patients with genotype 1-6 HCV infection treated with sofosbuvir/velpatasvir in the phase III studies. J Hepatol. 2018;68:895–903. - PubMed
-
- European Association for the Study of the Liver. European Association for the Study of the Liver EASL recommendations on treatment of hepatitis C 2018. J Hepatol. 2018;69:461–511. - PubMed
-
- American Association for the Study of Liver Diseases and the Infectious Diseases Society of America (AASLD) HCV guidance: recommendations for testing, managing, and treating hepatitis C. AASLD web site, < http://www.hcvguidelines.org/>. Accessed 15 Dec 2019. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous