Detection of enteric parasite DNA in household and bed dust samples: potential for infection transmission
- PMID: 32188497
- PMCID: PMC7079405
- DOI: 10.1186/s13071-020-04012-6
Detection of enteric parasite DNA in household and bed dust samples: potential for infection transmission
Abstract
Background: Enteric parasites are transmitted in households but few studies have sampled inside households for parasites and none have used sensitive molecular methods.
Methods: We collected bed and living room dust samples from households of children participating in a clinical trial of anthelmintic treatment in rural coastal Ecuador. Dust was examined for presence of DNA specific for 11 enteric parasites (Ascaris lumbricoides, Trichuris trichiura, Ancylostoma duodenale, Necator americanus, Strongyloides stercoralis, Toxocara canis and T. cati, Giardia lamblia, Blastocystis hominis, Cryptosporidium spp., and Entamoeba histolytica) by quantitative PCR (qPCR).
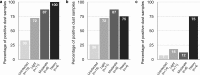
Results: Of the 38 households sampled, 37 had positive dust for at least one parasite and up to 8 parasites were detected in single samples. Positivity was greatest for B. hominis (79% of household samples) indicating a high level of environmental fecal contamination. Dust positivity rates for individual pathogens were: S. stercoralis (52%), A. lumbricoides (39%), G. lamblia (39%), Toxocara spp. (42%), hookworm (18%) and T. trichiura (8%). DNA for Cryptosporidium spp. and E. histolytica was not detected. Bed dust was more frequently positive than floor samples for all parasites detected. Positivity for A. lumbricoides DNA in bed (adjusted OR: 10.0, 95% CI: 2.0-50.1) but not floor dust (adjusted OR: 3.6, 95% CI: 0.3-37.9) was significantly associated with active infections in children.
Conclusions: To our knowledge, this is the first use of qPCR on environmental samples to detect a wide range of enteric pathogen DNA. Our results indicate widespread contamination of households with parasite DNA and raise the possibility that beds, under conditions of overcrowding in a humid tropical setting, may be a source of transmission.
Keywords: Beds; Dust; Ecuador; Enteric parasites; Environment; Floors; Protozoa; Soil-transmitted helminths; Transmission.
Conflict of interest statement
RM has received grant support from Romark Laboratiories. VSH, DGR, EC, AL and PJC declare that they have no competing interests.
Figures

Similar articles
-
Identification of human intestinal parasites affecting an asymptomatic peri-urban Argentinian population using multi-parallel quantitative real-time polymerase chain reaction.Parasit Vectors. 2015 Jul 17;8:380. doi: 10.1186/s13071-015-0994-z. Parasit Vectors. 2015. PMID: 26183074 Free PMC article.
-
Comparison of commercial and in-house real-time PCR platforms for 15 parasites and microsporidia in human stool samples without a gold standard.Acta Trop. 2020 Jul;207:105516. doi: 10.1016/j.actatropica.2020.105516. Epub 2020 May 3. Acta Trop. 2020. PMID: 32371221
-
A novel, multi-parallel, real-time polymerase chain reaction approach for eight gastrointestinal parasites provides improved diagnostic capabilities to resource-limited at-risk populations.Am J Trop Med Hyg. 2013 Jun;88(6):1041-7. doi: 10.4269/ajtmh.12-0726. Epub 2013 Mar 18. Am J Trop Med Hyg. 2013. PMID: 23509117 Free PMC article.
-
Diagnóstico molecular de parasitosis intestinales.Enferm Infecc Microbiol Clin (Engl Ed). 2020 Jan;38 Suppl 1:24-31. doi: 10.1016/j.eimc.2020.02.005. Enferm Infecc Microbiol Clin (Engl Ed). 2020. PMID: 32111362 Review. English, Spanish.
-
Prevalence of intestinal parasitic infections among communities living in different habitats and its comparison with one hundred and one studies conducted over the past 42 years (1970 to 2013) in Malaysia.Trop Biomed. 2014 Jun;31(2):190-206. Trop Biomed. 2014. PMID: 25134888 Review.
Cited by
-
Molecular Testing of Environmental Samples as a Potential Source to Estimate Parasite Infection.Trop Med Infect Dis. 2024 Sep 26;9(10):226. doi: 10.3390/tropicalmed9100226. Trop Med Infect Dis. 2024. PMID: 39453253 Free PMC article.
-
Better floors, better health: a theory of change for an improved household flooring intervention in rural communities in Kwale and Bungoma counties, Kenya.BMC Public Health. 2025 Feb 17;25(1):639. doi: 10.1186/s12889-025-21469-1. BMC Public Health. 2025. PMID: 39962442 Free PMC article. Clinical Trial.
-
Improved household flooring is associated with lower odds of enteric and parasitic infections in low- and middle-income countries: A systematic review and meta-analysis.PLOS Glob Public Health. 2023 Dec 1;3(12):e0002631. doi: 10.1371/journal.pgph.0002631. eCollection 2023. PLOS Glob Public Health. 2023. PMID: 38039279 Free PMC article.
-
Prevalence and associated risk factors of intestinal parasites among schoolchildren in Ecuador, with emphasis on the molecular diversity of Giardia duodenalis, Blastocystis sp. and Enterocytozoon bieneusi.PLoS Negl Trop Dis. 2023 May 24;17(5):e0011339. doi: 10.1371/journal.pntd.0011339. eCollection 2023 May. PLoS Negl Trop Dis. 2023. PMID: 37224177 Free PMC article.
-
Environmental Detection of Parasites in the Marginalized Paiute Reservations Compared to a Nearby Area.Am J Trop Med Hyg. 2024 Feb 13;110(3):457-459. doi: 10.4269/ajtmh.23-0712. Print 2024 Mar 6. Am J Trop Med Hyg. 2024. PMID: 38350146 Free PMC article.
References
-
- Vaz Nery S, Pickering AJ, Abate E, Asmare A, Barrett L, Benjamin-Chung J, et al. The role of water, sanitation and hygiene interventions in reducing soil-transmitted helminths: interpreting the evidence and identifying next steps. Parasit Vectors. 2019;12:273. doi: 10.1186/s13071-019-3532-6. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

