Age-stratified burden of pneumococcal community acquired pneumonia in hospitalised Canadian adults from 2010 to 2015
- PMID: 32188585
- PMCID: PMC7078693
- DOI: 10.1136/bmjresp-2019-000550
Age-stratified burden of pneumococcal community acquired pneumonia in hospitalised Canadian adults from 2010 to 2015
Abstract
Background: In Canada, 13-valent pneumococcal conjugate vaccine (PCV13) is recommended in childhood, in individuals at high risk of invasive pneumococcal disease (IPD) and in healthy adults aged ≥65 years for protection against vaccine-type IPD and pneumococcal community-acquired pneumonia (pCAP). Since vaccine recommendations in Canada include both age-based and risk-based guidance, this study aimed to describe the burden of vaccine-preventable pCAP in hospitalised adults by age.
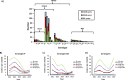
Methods: Surveillance for community-acquired pneumonia (CAP) in hospitalised adults was performed prospectively from 2010 to 2015. CAP was radiologically confirmed, and pCAP was identified using blood and sputum culture and urine antigen testing. Patient demographics and outcomes were stratified by age (16-49, 50-64, ≥65 and ≥50 years).
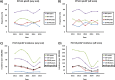
Results: Of 6666/8802 CAP cases tested, 830 (12.5%) had pCAP, and 418 (6.3%) were attributed to a PCV13 serotype. Of PCV13 pCAP, 41% and 74% were in adults aged ≥65 and ≥50 years, respectively. Compared with non-pCAP controls, pCAP cases aged ≥50 years were more likely to be admitted to intensive care units (ICUs) and to require mechanical ventilation. Older adults with pCAP were less likely to be admitted to ICU or required mechanical ventilation, given their higher mortality and goals of care. Of pCAP deaths, 67% and 90% were in the ≥65 and ≥50 age cohorts, respectively.
Conclusions: Adults hospitalised with pCAP in the age cohort of 50-64 years contribute significantly to the burden of illness, suggesting that an age-based recommendation for adults aged ≥50 years should be considered in order to optimise the impact of pneumococcal vaccination programmes in Canada.
Keywords: adult; burden; community-acquired pneumonia (CAP); pneumococcal vaccine, PCV13, ageStreptococcus pneumoniae; serotype.
© Author(s) (or their employer(s)) 2020. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: SAM received research grants and personal fees from GlaxoSmithKline, Pfizer and Sanofi Pasteur and personal fees from Merck; JL and TH participated on investigator-initiated grants sponsored by GSK and Pfizer; JL received grants from Merck; LV received research grants from GlaxoSmithKline, Pfizer, Optimer, Cubist and Merck, and personal fees from Merck, Optimer and Cubist. ML received grants from Pfizer. CL was a former employee for Pfizer Canada, Kirkland Québec, Québec. No other conflicts were declared.
Figures


References
-
- National Advisory Committee on immunization (NACI). Available: https://www.canada.ca/en/public-health/services/immunization/national-ad... [Accessed Dec 2019].
-
- Public Agency of Canada (PHAC) National laboratory surveillance of invasive streptococcal disease in Canada. annual summary, 2017. Available: https://www.canada.ca/en/public-health/services/publications/drugs-healt... [Accessed Dec 2019].
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
