Brain manganese and the balance between essential roles and neurotoxicity
- PMID: 32188696
- PMCID: PMC7212623
- DOI: 10.1074/jbc.REV119.009453
Brain manganese and the balance between essential roles and neurotoxicity
Abstract
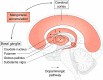
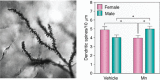
Manganese (Mn) is an essential micronutrient required for the normal development of many organs, including the brain. Although its roles as a cofactor in several enzymes and in maintaining optimal physiology are well-known, the overall biological functions of Mn are rather poorly understood. Alterations in body Mn status are associated with altered neuronal physiology and cognition in humans, and either overexposure or (more rarely) insufficiency can cause neurological dysfunction. The resultant balancing act can be viewed as a hormetic U-shaped relationship for biological Mn status and optimal brain health, with changes in the brain leading to physiological effects throughout the body and vice versa. This review discusses Mn homeostasis, biomarkers, molecular mechanisms of cellular transport, and neuropathological changes associated with disruptions of Mn homeostasis, especially in its excess, and identifies gaps in our understanding of the molecular and biochemical mechanisms underlying Mn homeostasis and neurotoxicity.
Keywords: brain; homeostasis; manganese; metal; metal homeostasis; neurodegeneration; neurodegenerative disease; neurodevelopment; neurotoxin; neurotransmitter; toxicology.
© 2020 Balachandran et al.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures


References
-
- Keen C. L., Ensunsa J. L., and Clegg M. S. (2000) Manganese metabolism in animals and humans including the toxicity of manganese. Met. Ions Biol. Syst. 37, 89–121 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

