Design of anti-inflammatory heparan sulfate to protect against acetaminophen-induced acute liver failure
- PMID: 32188725
- PMCID: PMC7315409
- DOI: 10.1126/scitranslmed.aav8075
Design of anti-inflammatory heparan sulfate to protect against acetaminophen-induced acute liver failure
Abstract
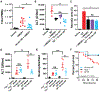
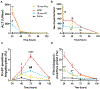
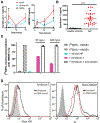
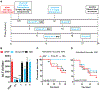
Acetaminophen/paracetamol (APAP) overdose is the leading cause of drug-induced acute liver failure (ALF) in the United States and Europe. The progression of the disease is attributed to sterile inflammation induced by the release of high mobility group box 1 (HMGB1) and the interaction with receptor for advanced glycation end products (RAGE). A specific, effective, and safe approach to neutralize the proinflammatory activity of HMGB1 is highly desirable. Here, we found that a heparan sulfate (HS) octadecasaccharide (18-mer-HP or hepatoprotective 18-mer) displays potent hepatoprotection by targeting the HMGB1/RAGE axis. Endogenous HS proteoglycan, syndecan-1, is shed in response to APAP overdose in mice and humans. Furthermore, purified syndecan-1, but not syndecan-1 core protein, binds to HMGB1, suggesting that HMGB1 binds to HS polysaccharide side chains of syndecan-1. Last, we compared the protection effect between 18-mer-HP and N-acetyl cysteine, which is the standard of care to treat APAP overdose. We demonstrated that 18-mer-HP administered 3 hours after a lethal dose of APAP is fully protective; however, the treatment of N-acetyl cysteine loses protection. Therefore, 18-mer-HP may offer a potential therapeutic advantage over N-acetyl cysteine for late-presenting patients. Synthetic HS provides a potential approach for the treatment of APAP-induced ALF.
Copyright © 2020 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works.
Conflict of interest statement
Figures



References
-
- Zitvogel L, Kepp O, Kroemer G, Decoding cell death signals in inflammation and immunity. Cell 140, 798–804 (2010). - PubMed
-
- Bianchi ME, Crippa MP, Manfredi AA, Mezzapelle R, Querini PR, Venereau E, High-mobility group box 1 protein orchestrates responses to tissue damage via inflammation, innate and adaptive immunity, and tissue repair. Immunol. Rev 280, 74–82 (2017). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R44 GM134738/GM/NIGMS NIH HHS/United States
- R01 DK111958/DK/NIDDK NIH HHS/United States
- R01 HL125371/HL/NHLBI NIH HHS/United States
- R01 HL142604/HL/NHLBI NIH HHS/United States
- R01 HL094463/HL/NHLBI NIH HHS/United States
- R44 HL139187/HL/NHLBI NIH HHS/United States
- P30 DK056350/DK/NIDDK NIH HHS/United States
- R42 GM128484/GM/NIGMS NIH HHS/United States
- R01 AR070179/AR/NIAMS NIH HHS/United States
- R41 GM123792/GM/NIGMS NIH HHS/United States
- R01 HL144970/HL/NHLBI NIH HHS/United States
- U01 GM102137/GM/NIGMS NIH HHS/United States
- R41 HL139187/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

