Evaluation of bile salt hydrolase inhibitor efficacy for modulating host bile profile and physiology using a chicken model system
- PMID: 32188876
- PMCID: PMC7080769
- DOI: 10.1038/s41598-020-61723-7
Evaluation of bile salt hydrolase inhibitor efficacy for modulating host bile profile and physiology using a chicken model system
Abstract
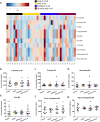
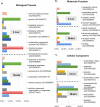
Gut microbial enzymes, bile salt hydrolases (BSHs) are the gateway enzymes for bile acid (BA) modification in the gut. This activity is a promising target for developing innovative non-antibiotic growth promoters to enhance animal production and health. Compelling evidence has shown that inhibition of BSH activity should enhance weight gain by altering the BA pool, host signalling and lipid metabolism. We recently identified a panel of promising BSH inhibitors. Here, we address the potential of them as alternative, effective, non-antibiotic feed additives, for commercial application, to promote animal growth using a chicken model. In this study, the in vivo efficacy of three BSH inhibitors (caffeic acid phenethylester, riboflavin, carnosic acid) were evaluated. 7-day old chicks (10 birds/group) were either untreated or they received one of the specific BSH inhibitors (25 mg/kg body weight) via oral gavage for 17 days. The chicks in treatment groups consistently displayed higher body weight gain than the untreated chicks. Metabolomic analysis demonstrated that BSH inhibitor treatment led to significant changes in both circulating and intestinal BA signatures in support of blunted intestinal BSH activity. Consistent with this finding, liver and intestinal tissue RNA-Seq analysis showed that carnosic acid treatment significantly altered expression of genes involved in lipid and bile acid metabolism. Taken together, this study validates microbial BSH activity inhibition as an alternative target and strategy to antibiotic treatment for animal growth promotion.
Conflict of interest statement
The authors declare no competing interests.
Figures







References
-
- Moore P, et al. Use of sulfasuxidine, streptothricin, and streptomycin in nutritional studies with the chick. J. Biol. Chem. 1946;165:437–441. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

