Novel Chemo-Photothermal Therapy in Breast Cancer Using Methotrexate-Loaded Folic Acid Conjugated Au@SiO2 Nanoparticles
- PMID: 32189075
- PMCID: PMC7080937
- DOI: 10.1186/s11671-020-3295-1
Novel Chemo-Photothermal Therapy in Breast Cancer Using Methotrexate-Loaded Folic Acid Conjugated Au@SiO2 Nanoparticles
Abstract
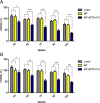
Low level laser therapy (LLLT) is known as a safe type of phototherapy to target tumor tissue/cells. Besides, using targeted nanoparticles increases the successfulness of cancer therapy. This study was designed for investigating the combined effect of folate (FA)/Methotrexate (MTX) loaded silica coated gold (Au@SiO2) nanoparticles (NPs) and LLLT on the fight against breast cancer.NPs were synthesized and characterized using FTIR, TEM and DLS-Zeta. The NPs had spherical morphology with mean diameter of around 25 nm and positive charge (+13.3 mV) while after conjugation with FA and MTX their net charge reduced to around -19.7 mV.Our findings in cell uptake studies clearly showed enhanced cellular uptake of NPs after FA and MTX loaded NPs in both breast cancer cell lines especially on MDA-MB-231 due to high expression of folate receptors. The results indicated that LLLT had a proliferative effect on both breast cancer cell lines but in the presence of engineered breast cancer targeted nanoparticle, the efficacy of combination chemo-photothermal therapy was significantly increased using MTT assay (p<0.05), DAPI staining, and cell cycle findings. The highest apoptotic effect on breast cancer cell lines was observed in the cells exposed to a combination of MTX-FA loaded Au@SiO2 NP and LLLT proved by DAPI staining and cell cycle(by increasing the cell arrest in subG0/G1). Taken together a combination of chemotherapy and LLLT improves the potential of breast cancer therapy with minimum side effects.
Keywords: Au@SiO2 nanoparticles; Breast cancer; Folic acid; Low level laser therapy; Methotrexate.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures








References
LinkOut - more resources
Full Text Sources
Miscellaneous

