Downregulation of SFRP1 is a protumorigenic event in hepatoblastoma and correlates with beta-catenin mutations
- PMID: 32189106
- PMCID: PMC7142044
- DOI: 10.1007/s00432-020-03182-1
Downregulation of SFRP1 is a protumorigenic event in hepatoblastoma and correlates with beta-catenin mutations
Abstract
Background: Hepatoblastoma (HB) and pediatric hepatocellular carcinoma (HCC) are the most common malignant liver tumors in childhood. Both tumor types exhibit genetic and epigenetic alterations in the WNT/β-catenin signaling pathway, which is a key regulator of liver progenitor cells in embryonic development. The tumors demonstrate a high rate of β-catenin mutations and gene expression changes of several WNT antagonists. However, the role of the WNT inhibitory factor secreted frizzled-related protein 1 (SFRP1) has not been addressed in pediatric liver cancer so far.
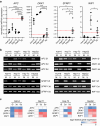
Results: In our study, we investigated the gene expression level, DNA methylation status and functional relevance of SFRP1 in HB cell lines and in pediatric liver tumor patient samples. SFRP1 was downregulated due to DNA promoter methylation in all tested HB cell lines. Overexpression of SFRP1 in HB cell lines diminished tumor cell proliferation, colony formation and migration potential. In addition, the SFRP1-expressing HB cell lines showed reduced WNT/β-catenin signaling pathway activity and decreased expression of WNT target genes. To evaluate the utility of SFRP1 as a biomarker in pediatric liver cancer, we determined the gene expression level and DNA methylation status of SFRP1 in 45 pediatric liver tumor patient samples. The correlation analysis of different clinical parameters and tumor characteristics revealed a significant correlation of reduced SFRP1 expression with the presence of mutant β-catenin. The methylation status of SFRP1 was furthermore associated to a pediatric liver tumor type with HCC-like characteristics, TERT mutations and an older age at diagnosis.
Conclusion: Altogether, our data demonstrate that the epigenetic suppression of the WNT/β-catenin antagonist SFRP1 has an important impact on the malignant behavior of HB cells. Although SFRP1 methylation is a common event in HCC-like pediatric liver tumors, its potential as a prognostic or diagnostic biomarker needs to be further investigated.
Keywords: APC; DKK1; DNA methylation; Epigenetics; Hepatoblastoma; Hepatocellular carcinoma; Pediatric liver cancer; SFRP1; WIF1; WNT signaling.
Conflict of interest statement
The authors declare that they have no conflict of interests.
Figures


Similar articles
-
Epigenetic inactivation of the canonical Wnt antagonist secreted frizzled-related protein 1 in hepatocellular carcinoma cells.Neoplasma. 2012;59(3):326-32. doi: 10.4149/neo_2012_042. Neoplasma. 2012. PMID: 22296502
-
Epigenetic silencing of sFRP1 activates the canonical Wnt pathway and contributes to increased cell growth and proliferation in hepatocellular carcinoma.Tumour Biol. 2012 Apr;33(2):325-36. doi: 10.1007/s13277-012-0331-5. Epub 2012 Feb 15. Tumour Biol. 2012. PMID: 22351518
-
Hepatitis C virus core protein epigenetically silences SFRP1 and enhances HCC aggressiveness by inducing epithelial-mesenchymal transition.Oncogene. 2014 May 29;33(22):2826-35. doi: 10.1038/onc.2013.225. Epub 2013 Jun 17. Oncogene. 2014. PMID: 23770846
-
Integrative analysis of aberrant Wnt signaling in hepatitis B virus-related hepatocellular carcinoma.World J Gastroenterol. 2015 May 28;21(20):6317-28. doi: 10.3748/wjg.v21.i20.6317. World J Gastroenterol. 2015. PMID: 26034368 Free PMC article. Review.
-
Wnt/β-catenin signaling as a useful therapeutic target in hepatoblastoma.Biosci Rep. 2019 Sep 24;39(9):BSR20192466. doi: 10.1042/BSR20192466. Print 2019 Sep 30. Biosci Rep. 2019. PMID: 31511432 Free PMC article. Review.
Cited by
-
Concentration of Secreted Frizzled-Related Proteins (SFRPs) in Non-Small Cell Lung Carcinoma Subtypes-A Preliminary Study.Curr Oncol. 2023 Nov 18;30(11):9968-9980. doi: 10.3390/curroncol30110724. Curr Oncol. 2023. PMID: 37999144 Free PMC article.
-
Cross-talk between cancer stem cells and immune cells: potential therapeutic targets in the tumor immune microenvironment.Mol Cancer. 2023 Feb 21;22(1):38. doi: 10.1186/s12943-023-01748-4. Mol Cancer. 2023. PMID: 36810098 Free PMC article. Review.
-
miR-126 in Extracellular Vesicles Derived from Hepatoblastoma Cells Promotes the Tumorigenesis of Hepatoblastoma through Inducing the Differentiation of BMSCs into Cancer Stem Cells.J Immunol Res. 2021 Oct 29;2021:6744715. doi: 10.1155/2021/6744715. eCollection 2021. J Immunol Res. 2021. PMID: 34746322 Free PMC article.
-
Hypoxic Non-Small-Cell Lung Cancer Cell-Secreted Exosomal microRNA-582-3p Drives Cancer Cell Malignant Phenotypes by Targeting Secreted Frizzled-Related Protein 1.Cancer Manag Res. 2020 Oct 14;12:10151-10161. doi: 10.2147/CMAR.S263768. eCollection 2020. Cancer Manag Res. 2020. PMID: 33116870 Free PMC article.
-
Targeting Epigenetic Modifiers of Tumor Plasticity and Cancer Stem Cell Behavior.Cells. 2022 Apr 21;11(9):1403. doi: 10.3390/cells11091403. Cells. 2022. PMID: 35563709 Free PMC article. Review.
References
-
- Aguilera O, Fraga MF, Ballestar E, Paz MF, Herranz M, Espada J et al (2006) Epigenetic inactivation of the Wnt antagonist DICKKOPF-1 (DKK-1) gene in human colorectal cancer. Oncogene 25(29):4116–4121 - PubMed
-
- Anastas JN, Moon RT (2013) WNT signalling pathways as therapeutic targets in cancer. Nat Rev Cancer 13(1):11–26 - PubMed
-
- Atschekzei F, Hennenlotter J, Janisch S, Grosshennig A, Trankenschuh W, Waalkes S et al (2012) SFRP1 CpG Island methylation locus is associated with renal cell cancer susceptibility and disease recurrence. Epigenetics 7(5):447–457 - PubMed
-
- Ba S, Xuan Y, Long ZW, Chen HY, Zheng SS (2017) MicroRNA-27a promotes the proliferation and invasiveness of colon cancer cells by targeting SFRP1 through the Wnt/β-catenin signaling pathway. Cell Physiol Biochem 42(5):1920–1933 - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

