'Minimal symptom expression' in patients with acetylcholine receptor antibody-positive refractory generalized myasthenia gravis treated with eculizumab
- PMID: 32189108
- PMCID: PMC7320935
- DOI: 10.1007/s00415-020-09770-y
'Minimal symptom expression' in patients with acetylcholine receptor antibody-positive refractory generalized myasthenia gravis treated with eculizumab
Abstract
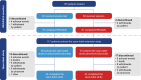
Background: The efficacy and tolerability of eculizumab were assessed in REGAIN, a 26-week, phase 3, randomized, double-blind, placebo-controlled study in anti-acetylcholine receptor antibody-positive (AChR+) refractory generalized myasthenia gravis (gMG), and its open-label extension.
Methods: Attainment of 'minimal symptom expression' was evaluated using patient-reported outcome measures of gMG symptoms [MG activities of daily living scale (MG-ADL), 15-item MG quality of life questionnaire (MG-QOL15)] at the completion of REGAIN and during the open-label extension. 'Minimal symptom expression' was defined as MG-ADL total score of 0-1 or MG-QOL15 total score of 0-3.
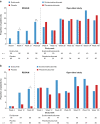
Results: At REGAIN week 26, more eculizumab-treated patients achieved 'minimal symptom expression' versus placebo [MG-ADL: 21.4% vs 1.7%; difference 19.8%; 95% confidence interval (CI) 8.5, 31.0; p = 0.0007; MG-QOL15: 16.1% vs 1.7%; difference 14.4%; 95% CI 4.3, 24.6; p = 0.0069]. During the open-label extension, the proportion of patients in the placebo/eculizumab group who achieved 'minimal symptom expression' increased after initiating eculizumab treatment and was sustained through 130 weeks of open-label eculizumab (MG-ADL: 1.7 to 27.8%; MG-QOL15: 1.7 to 19.4%). At extension study week 130, similar proportions of patients in the eculizumab/eculizumab and placebo/eculizumab groups achieved 'minimal symptom expression' (MG-ADL: 22.9% and 27.8%, respectively, p = 0.7861; MG-QOL15: 14.3% and 19.4%, respectively, p = 0.7531). The long-term tolerability of eculizumab was consistent with previous reports.
Conclusions: Patients with AChR+ refractory gMG who receive eculizumab can achieve sustained 'minimal symptom expression' based on patient-reported outcomes. 'Minimal symptom expression' may be a useful tool in measuring therapy effectiveness in gMG.
Trial registration: ClinicalTrials.gov NCT01997229, NCT02301624.
Keywords: Acetylcholine receptor; Eculizumab; Minimal symptom expression; Myasthenia gravis; Refractory.
Conflict of interest statement
This work was funded by Alexion Pharmaceuticals. J.V. has received research and travel support, and/or speaker honoraria from Alexion Pharmaceuticals and Sanofi/Genzyme, and has served on advisory boards or as a consultant for Asklepios Biopharmaceuticals, Audentes Therapeutics, Novartis Pharma AG, PTC Therapeutics, Roche, Sanofi/Genzyme, Santhera Pharmaceuticals, Sarepta Therapeutics, and Stealth Biotherapeutics within the past 3 years. S.J. is a member of an international advisory board for Alexion Pharmaceuticals, has been an advisory board member for Alnylam Pharmaceuticals and Argenx BVBA, has received speaker fees from Terumo BCT, and has received research support from the Wellcome Trust Clinical Research Facility and Centre for Rare Diseases at the University Hospitals Birmingham, UK. K.P.F. was employed by and owns stock in Alexion Pharmaceuticals and is employed by Alnylam Pharmaceuticals. F.O’B. is employed by, and owns stock in, Alexion Pharmaceuticals. J.F.H. has received research support from Alexion Pharmaceuticals, argenx BVBA, the Centers for Disease Control and Prevention (Atlanta, GA, USA), the Muscular Dystrophy Association, the National Institutes of Health (including the National Institute of Neurological Disorders and Stroke and the National Institute of Arthritis and Musculoskeletal and Skin Diseases) and Ra Pharmaceuticals; has received honoraria from Alexion Pharmaceuticals; and has received non-financial support from Alexion Pharmaceuticals, argenx BVBA, Ra Pharmaceuticals and Toleranzia.
Figures
References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

