Biological phase separation: cell biology meets biophysics
- PMID: 32189162
- PMCID: PMC7242575
- DOI: 10.1007/s12551-020-00680-x
Biological phase separation: cell biology meets biophysics
Abstract
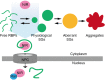
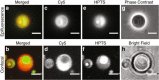
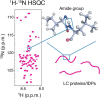
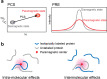
Progress in development of biophysical analytic approaches has recently crossed paths with macromolecule condensates in cells. These cell condensates, typically termed liquid-like droplets, are formed by liquid-liquid phase separation (LLPS). More and more cell biologists now recognize that many of the membrane-less organelles observed in cells are formed by LLPS caused by interactions between proteins and nucleic acids. However, the detailed biophysical processes within the cell that lead to these assemblies remain largely unexplored. In this review, we evaluate recent discoveries related to biological phase separation including stress granule formation, chromatin regulation, and processes in the origin and evolution of life. We also discuss the potential issues and technical advancements required to properly study biological phase separation.
Keywords: Intrinsically disordered region/protein (IDR/IDP); Liquid-liquid phase separation (LLPS); Low-complexity (LC) domain; Membrane-less organelle.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures





Similar articles
-
Biological soft matter: intrinsically disordered proteins in liquid-liquid phase separation and biomolecular condensates.Essays Biochem. 2022 Dec 16;66(7):831-847. doi: 10.1042/EBC20220052. Essays Biochem. 2022. PMID: 36350034 Review.
-
Phase Separation of Epstein-Barr Virus EBNA2 and Its Coactivator EBNALP Controls Gene Expression.J Virol. 2020 Mar 17;94(7):e01771-19. doi: 10.1128/JVI.01771-19. Print 2020 Mar 17. J Virol. 2020. PMID: 31941785 Free PMC article.
-
Liquid-liquid phase separation in tumor biology.Signal Transduct Target Ther. 2022 Jul 8;7(1):221. doi: 10.1038/s41392-022-01076-x. Signal Transduct Target Ther. 2022. PMID: 35803926 Free PMC article. Review.
-
Liquid-Liquid Phase Separation in Biology: Specific Stoichiometric Molecular Interactions vs Promiscuous Interactions Mediated by Disordered Sequences.Biochemistry. 2021 Aug 10;60(31):2397-2406. doi: 10.1021/acs.biochem.1c00376. Epub 2021 Jul 22. Biochemistry. 2021. PMID: 34291921 Review.
-
Liquid-liquid phase separation (LLPS) in cellular physiology and tumor biology.Am J Cancer Res. 2021 Aug 15;11(8):3766-3776. eCollection 2021. Am J Cancer Res. 2021. PMID: 34522448 Free PMC article. Review.
Cited by
-
All-trans retinoic acid improves NSD2-mediated RARα phase separation and efficacy of anti-CD38 CAR T-cell therapy in multiple myeloma.J Immunother Cancer. 2023 Mar;11(3):e006325. doi: 10.1136/jitc-2022-006325. J Immunother Cancer. 2023. PMID: 36918219 Free PMC article.
-
Membraneless organelles restructured and built by pandemic viruses: HIV-1 and SARS-CoV-2.J Mol Cell Biol. 2021 Aug 4;13(4):259-268. doi: 10.1093/jmcb/mjab020. J Mol Cell Biol. 2021. PMID: 33760045 Free PMC article. Review.
-
Catalytic RNA Oligomers Formed by Co-Oligomerization of a Pair of Bimolecular RNase P Ribozymes.Molecules. 2022 Nov 28;27(23):8298. doi: 10.3390/molecules27238298. Molecules. 2022. PMID: 36500390 Free PMC article.
-
Super-enhancer-associated TMEM44-AS1 aggravated glioma progression by forming a positive feedback loop with Myc.J Exp Clin Cancer Res. 2021 Oct 25;40(1):337. doi: 10.1186/s13046-021-02129-9. J Exp Clin Cancer Res. 2021. PMID: 34696771 Free PMC article.
-
Environmental Stability and Its Importance for the Emergence of Darwinian Evolution.Life (Basel). 2023 Sep 25;13(10):1960. doi: 10.3390/life13101960. Life (Basel). 2023. PMID: 37895342 Free PMC article.
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

