Hemoglobin: Structure, Function and Allostery
- PMID: 32189307
- PMCID: PMC7370311
- DOI: 10.1007/978-3-030-41769-7_14
Hemoglobin: Structure, Function and Allostery
Abstract
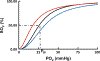
This chapter reviews how allosteric (heterotrophic) effectors and natural mutations impact hemoglobin (Hb) primary physiological function of oxygen binding and transport. First, an introduction about the structure of Hb is provided, including the ensemble of tense and relaxed Hb states and the dynamic equilibrium of Hb multistate. This is followed by a brief review of Hb variants with altered Hb structure and oxygen binding properties. Finally, a review of different endogenous and exogenous allosteric effectors of Hb is presented with particular emphasis on the atomic interactions of synthetic ligands with altered allosteric function of Hb that could potentially be harnessed for the treatment of diseases.
Keywords: Allosteric effectors; Allostery; Hemoglobin; Hemoglobin variants; Oxygen affinty; Relaxed state; T state; X-ray crystallography.
Conflict of interest statement
Figures

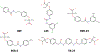
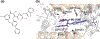
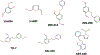

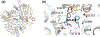

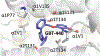
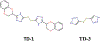
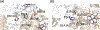
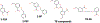
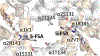
References
-
- Abdulmalik O, Ghatge MS, Musayev FN et al. (2011) Crystallographic analysis of human hemoglobin elucidates the structural basis of the potent and dual antisickling activity of pyridyl derivatives of vanillin Corrigendum. Acta Crystallogr D Biol Crystallogr 67:1076 10.1107/S0907444911045860 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

